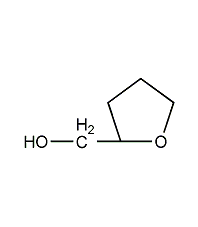
Structural formula
| Business number | 02D0 |
|---|---|
| Molecular formula | C5H10O2 |
| Molecular weight | 102.13 |
| label |
Tetrahydrofuranmethanol, Tetrahydro-2-furanmethanol, Tetrahydro-2-furanylmethanol, Tetrahydro-2-furanmethanol, Gelatin solution stabilizer, Wetting agent for printing and dyeing industry, Dispersant, Decolorizing and deodorizing agents for certain drugs, synthetic raw materials, Intermediates |
Numbering system
CAS number:97-99-4
MDL number:MFCD00005372
EINECS number:202-625-6
RTECS number:LU2450000
BRN number:102723
PubChem number:24888894
Physical property data
1. Properties: Colorless and transparent liquid with a slightly pleasant odor, which gradually becomes darker when exposed to air and is hygroscopic.
2. Boiling point (ºC, 101.3kPa): 178
3. Melting point (ºC): -80
4. Relative density (g/mL, 25/4ºC): 1.0524
5. Relative density (g/mL, 31ºC): 1.0402
6. Relative vapor density (g/mL, air=1): 3.5
7. Refractive index (20ºC): 1.4520
8. Refractive index (25ºC): 1.4499
9. Viscosity (mPa·s, 20ºC): 6.24
10. Flash point (ºC, open): 83
11. Fire point (ºC): 282
12. Heat of vaporization (KJ/mol, 25ºC ): 51.58
13. Heat of evaporation (KJ/mol, b.p.): 45.22
14. Heat of combustion (KJ/mol): 2970.5
15. Specific heat capacity (KJ/(kg·K), 20~27ºC, constant pressure): 1.78
16. Vapor pressure (kPa, 25ºC): 0.107
17. Lower explosion limit (% ,V/V): 1.5
18. Explosion upper limit (%,V/V): 9.7
19. Volume expansion coefficient (K-1 ,20~37.8ºC): 0.00052
20. Solubility: Miscible with water, alcohol, ether, acetone, chloroform, benzene and other solvents. Can dissolve rosin, grease, shellac, coumarone resin, cellulose acetate, ethyl cellulose, benzyl cellulose, nitrocellulose, alkyd resin, furfuryl alcohol polymer, polyvinyl acetate, polystyrene, chlorine Chemical rubber, etc.
21. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -3027.5
22. Gas phase standard claimed heat (enthalpy) ( kJ·mol-1): -369.2
23. Liquid phase�� Quasi combustion heat (enthalpy) (kJ·mol-1): -2961.0
24. Liquid phase standard claims heat (enthalpy) (kJ·mol- 1): -435.7
25. Liquid phase standard entropy (J·mol-1·K-1): 219.2
26. Liquid phase standard hot melt (J·mol-1·K-1): 190
Toxicological data
1. Skin/eye irritation: Standard Dresser test: rabbit eye contact, 20mg/24HREACTION SEVERITY, moderate reaction; 2. Acute toxicity: rat oral LD50: 1600mg/kg; rat peritoneal cavity LD50: 400mg/kg ; The mouse passed the mouth LD50: 2300mg/kg; the rabbit intravenous injection LD50: 725mg/kg; the guinea pig LD50: 800mg/kg; the skin contact LD50: 5mg/kg of the fish; Causes moderate skin irritation, contact with skin should be avoided. When rats inhaled 2.74g/m3 for 6 hours, they developed ataxia and failure; when exposed to 52.9g/m3 for 6 hours, some animals died.
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 26.28
2. Molar volume (cm3/mol): 98.3
3. Isotonic specific volume (90.2K ): 245.8
4. Surface tension (dyne/cm): 39.0
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 10.41
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.1
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 7
8. Surface charge: 0
9. Complexity: 54
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with strong oxidants, strong reducing agents, strong acids, acid anhydrides, and acid chlorides.
2. Chemical properties: It has the chemical properties of primary alcohols. Dehydration produces 2,3-dihydropyran.
3. Exist in flue-cured tobacco leaves.
Storage method
Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container tightly sealed. They should be stored separately from oxidants, reducing agents, acids, acid anhydrides and acid chlorides, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Prepared by hydrogenation of furfural. When a nickel-chromium-copper catalyst is used, the reaction temperature is 170-180°C and the pressure is 7.5-10.5MPa. It is obtained by continuous pressurized hydrogenation of furfural in the presence of Ni catalyst at 170~180℃ and 7.355~7.845MPa.
Refining method: fractional distillation and refining.
2. Tobacco: FC, 9, 18.
Purpose
Used as solvent for grease, wax, resin, dye, cellulose acetate, cellulose nitrate, ethyl cellulose, etc. It is also used as a stabilizer for gelatin solutions, a wetting agent and dispersant in the printing and dyeing industry, and a decolorizing and deodorizing agent for certain pharmaceuticals. In addition, tetrahydrofurfuryl alcohol is also used to prepare dihydrofuran, lysine, polyamide plastics, plasticizers, etc.
extended-reading:https://www.newtopchem.com/archives/40475
extended-reading:https://www.cyclohexylamine.net/catalyst-sa-1-polyurethane-catalyst-sa-1/
extended-reading:https://www.newtopchem.com/archives/44661
extended-reading:https://www.bdmaee.net/fomrez-ul-38-catalyst-dioctyldodecyltin-oxide-momentive/
extended-reading:https://www.newtopchem.com/archives/category/products/page/103
extended-reading:https://www.newtopchem.com/archives/40405
extended-reading:https://www.newtopchem.com/archives/1045
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/1-1.jpg
extended-reading:https://www.bdmaee.net/dimethylbenzylamine-cas-103-83-3-n-dimthylbenzylamine/
extended-reading:https://www.bdmaee.net/dibutyltin-monooctyl-maleate/




















Comments