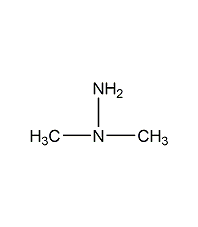
Structural formula
| Business number | 018F |
|---|---|
| Molecular formula | C2H8N2 |
| Molecular weight | 60.08 |
| label |
None |
Numbering system
CAS number:57-14-7
MDL number:MFCD00007628
EINECS number:200-316-0
RTECS number:MV2450000
BRN number:605261
PubChem number:24893497
Physical property data
1. Properties: Colorless liquid with ammonia odor, hygroscopic. [1]
2. Melting point (℃): -58[2]
3. Boiling point (℃): 63.9[3]
4. Relative density (water=1): 0.78 (25℃)[4]
5. Relative vapor density (air = 1): 2.1[5]
6. Saturated vapor pressure (kPa): 16.4 (20℃)[6]
7. Heat of combustion (kJ/mol): -1979[7]
8. Critical temperature (℃): 250[8]
9. Critical pressure (MPa): 5.42[9]
10. Octanol/water partition coefficient: -1.19[10]
11. Flash point (℃): -15 (CC) [11]
12 .Ignition temperature (℃): 249[12]
13. Explosion limit (%): 95[13]
14. Lower explosion limit (%): 2.0[14]
15. Solubility: miscible with water, miscible with dimethylformamide, ethanol, and ether ,hydrocarbon. [15]
16. Refractive index (25ºC): 1.4508
17. Ignition point (ºC): 249
18. Heat of evaporation (KJ/mol): 35.02
19. Heat of fusion (KJ/mol): 10.08
20. Heat of generation (KJ/mol): 49.37
Toxicological data
1. Acute toxicity[16]
LD50: 122mg/kg (rat oral); 1060mg/kg (rabbit dermal )
LC50: 252ppm (rat inhalation, 4h)
2. Irritation No information available
3 .Subacute and chronic toxicity [17] Dogs inhaled 12.5mg/m3, 6 hours a day, 5 times/week, 26 weeks, weight loss and lethargy , mild anemia.
4. Mutagenicity [18] Microbial mutagenicity: Salmonella typhimurium 42 μmol/dish. DNA repair: E. coli 600μg/dish. DNA damage: human fibroblasts 300 μmol/L.
5. Carcinogenicity [19] IARC Carcinogenicity Comment: G2B, suspected carcinogen in humans.
Ecological data
1. Ecotoxicity[20]
LC50: 11.35mg/L (96h) (channel catfish); 7.85mg/L ( 96h) (fathead minnow, 30d); 38mg/L (24h) (water fleas)
2. Biodegradability[21]
Aerobic biodegradation (h): 192~528
Anaerobic biodegradation (h): 768~2112
3. Abiotic degradation Properties[22]
Photooxidation half-life in air (h): 0.8~7.7
Molecular structure data
1. Molar refractive index: 18.57
2. Molar volume (cm3/mol): 72.4
3. Isotonic specific volume (90.2K ): 166.4
4. Surface tension (dyne/cm): 27.8
5. Polarizability (10-24cm3): 7.36
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): -0.5
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
p>
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 29.3
7. Number of heavy atoms: 4
8. Surface charge: 0
9. Complexity: 11.5
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Chemical properties: It has strong reducing properties. Contact with any oxidizing agent will cause combustion and explosion. Strongly hygroscopic. It reacts with acid to form salt; reacts with nitrous acid to form dimethylamine; reacts with aldehydes and ketones to form hydrazone.
2. Highly toxic and can cause cancer. After vapor is inhaled, irritation symptoms of the nasal cavity and throat, difficulty breathing, nausea, severe vomiting and neurological symptoms, neurasthenia, unsteady gait, convulsions, coma, etc. may occur. Eye manifestations include mild conjunctivitis. The oral LD50 of white mice is 265mg/kg. The time-weighted average allowable concentration of toxic substances in the air in the workplace is 0.5mg/m3; the allowable concentration for short-term exposure is 1.5mg/m3. There is no specific antidote for poisoning, only symptomatic treatment. The U.S. Occupational Safety and Health Administration stipulates that the maximum allowable exposure concentration in the air is 1mg/m3.
3. Stability[23] Stable
4. Incompatible substances[24] Oxidants, copper and its alloys, aluminum, iron, iron salts
5. Conditions to avoid contact [25] Heating
6. Polymerization hazard[26] No polymerization
Storage method
Storage Precautions[27] Store in a cool, well-ventilated special warehouse, and implement the "two people to send and receive, and two people to keep" system. Keep away from fire and heat sources. The storage temperature should not exceed 37℃. Keep container tightly sealed. They should be stored separately from oxidants, metal powders, and food chemicals, and avoid mixed storage. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. This product can be synthesized by reacting ammonia, amine chloride and dimethylamine as raw materials. First, ammonia water and sodium hypochlorite are respectively sent to the one-step reactor for reaction to generate chlorinated amine, and then the chlorinated amine is sent to the two-step reactor to be synthesized with the dimethylamine aqueous solution to generate an aqueous solution of undimethylhydrazine, and then the synthetic liquid is sent to Enter a series of distillation towers for further distillation and distillation to remove excess ammonia, dimethylamine and partial hydrazone. After adding alkali for concentration and degassing, the finished product is obtained. 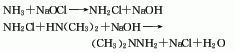
2. Preparation of N, N-dimethylhydrazine
In a 5-liter round-bottomed flask equipped with a mechanical stirrer, dropping funnel and thermometer, add 200 grams (2.7 moles) of nitrosodimethylamine, 3 liters of water and 650 grams (10 grams of atoms) 100% zinc powder. The reaction mixture was heated in a water bath to maintain the temperature at 25-30°C, and 1 liter (14 moles) of 85% acetic acid was added dropwise with stirring for about 2 hours. Then heat at 60°C for 1 hour, cool, filter out excess zinc powder, wash the combined aqueous solution with a small amount of water, place it in a 12-liter flask for steam distillation, install a dropping funnel on the flask, and add 1000 grams of hydroxide from the funnel The concentrated sodium solution makes the aqueous solution obviously alkaline, and steam distillation is carried out until the distillate has only a weak reducing effect on Fehling's solution. About 5 to 6 liters of distillate is enough to completely take out dimethylhydrazine.
After the distillate is treated with 650 ml of concentrated hydrochloric acid, it is concentrated under reduced pressure and on a steam bath until the residue becomes slurry. The slurry is dropped onto a large excess of solid sodium hydroxide, and then distilled until the temperature rises to 100°C, a concentrated aqueous solution of dimethylhydrazine can be obtained. If potassium hydroxide is added to the concentrated aqueous solution of dimethylhydrazine, left to dry, and distilled again, the distillate is collected in a receiver containing barium hydroxide, left for a few days, and then distilled to collect the 62~65°C/765mm fraction. That is anhydrous dimethylhydrazine. If the slurry is treated with absolute ethanol, white crystals of dimethylhydrazine hydrochloride can be obtained.
Purpose
1. This product is used to produce plant growth regulators, and its phenolate can reduce the deposition of lubricating salts. It can also be used to absorb acidic gases, and can also be used as analytical reagents, high-energy fuels, and solvents.
2. Carbonyl protecting reagent. Used in numerous ring-enlarging reactions, alkylation of N, N-dimethylhydrazone, monoalkylation of α, β-unsaturated ketones and conversion of aldehydes into nitriles.
3. Used in chemical synthesis, as a stabilizer of organic peroxides, acid gas absorbent, and also used in photography and agriculture. [28]
extended-reading:https://www.newtopchem.com/archives/626
extended-reading:https://www.bdmaee.net/rc-catalyst-108-cas108-39-4-rhine-chemical/
extended-reading:https://www.bdmaee.net/u-cat-sa-102-catalyst-cas112051-70-6-sanyo-japan/
extended-reading:https://www.cyclohexylamine.net/cas7560-83-0/
extended-reading:https://www.cyclohexylamine.net/nnnnn-pentamethyldiethylenetriamine-pmdeta/
extended-reading:https://www.bdmaee.net/fascat8201-catalyst/
extended-reading:https://www.bdmaee.net/fentacat-d89-catalyst-cas108-13-7-solvay/
extended-reading:https://www.bdmaee.net/butyltin-trichloridembtl/
extended-reading:https://www.newtopchem.com/archives/category/products/page/96
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-XD-104--tertiary-amine-catalyst-catalyst-XD-104.pdf




















Comments