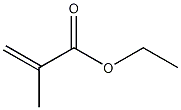
Structural formula
| Business number | 02CG |
|---|---|
| Molecular formula | C6H10O2 |
| Molecular weight | 114.14 |
| label |
Ethyl-2-methyl-2-acrylate, Ethyl methacrylate, Ethyl methacrylate, Ethyl methacrylate, 2-Ethyl methacrylate, Ethyl methacrylic acid, Ethyl methyl acrylate, Ethyl methacrylate, 2-Methyl-2-propenoic acid ethyl ester, 2-Methylacrylic acid ethyl ester, EMA, Methacrylic acid ethyl ester, aliphatic compounds |
Numbering system
CAS number:97-63-2
MDL number:MFCD00009161
EINECS number:202-597-5
RTECS number:OZ4550000
BRN number:471201
PubChem number:24884362
Physical property data
1. Properties: Colorless liquid, easily volatile, with spicy taste. [1]
2. Melting point (℃): -75[2]
3. Boiling point (℃): 117~119[3]
4. Relative density (water=1): 0.91 (25℃)[4]
5. Relative vapor density (air=1): 3.9[5]
6. Saturated vapor pressure (kPa): 2.0 (20℃)[6 ]
7. Heat of combustion (KJ/mol): -3356.3[7]
8. Critical pressure (MPa): 3.25 [8]
9. Octanol/water partition coefficient: 1.94[9]
10. Flash point (℃ ): 20 (OC) [10]
11. Ignition temperature (℃): 370[11]
12. Explosion upper limit (%): 9.6[12]
13. Explosion lower limit (%): 1.8[13]
14. Solubility: Slightly soluble in water, miscible in ethanol and ether. [14]
Toxicological data
1. Acute toxicity[15]
LD50: 14800mg/kg (rat oral)
LC50: 8300ppm (rat inhalation, 4h)
2. Irritation No information available
3. Others [16] The lowest toxic dose in the abdominal cavity of rats (TDLo): 735mg/kg (administered on 5th to 15th day of pregnancy), causing embryotoxicity.
Ecological data
1. Ecotoxicity No data available
2. Biodegradability No data available
3 .Non-biodegradable[17] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3, the degradation half-life is 19h (theoretical).
When the pH value is 11, the hydrolysis half-life is 2.5h.
4. Other harmful effects[18] This substance may be harmful to the environment, and special attention should be paid to water bodies.
Molecular structure data
1. Molar refractive index: 31.18
2. Molar volume (cm3/mol): 125.9
3. Isotonic specific volume (90.2K ): 280.7
4. Surface tension (dyne/cm): 24.6
5. Polarizability: 12.36
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.9
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 26.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 105
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. The toxicity of this product is similar to that of methyl methacrylate, and the oral LD50 is 15mL/kg. The protection requirements are the same as those for methyl methacrylate. See methyl methacrylate.
2. Stability[19] Stable
3. Incompatible substances[20] Strong oxidants, strong acids, strong alkali
4. Conditions to avoid contact [21] Heat, light, ultraviolet rays , contact with air
5. Polymerization hazard[22] Polymerization
Storage method
Storage Precautions[23] Usually products contain polymerization inhibitors. Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep away from light. The storage temperature should not exceed 37℃. The packaging must be sealed and must not come into contact with air. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
1. Obtained from the esterification of methacrylic acid and ethanol.
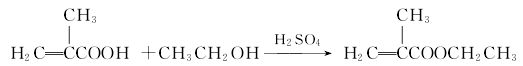
Combine methacrylic acid and anhydrous Add ethanol to the reaction pot, add a small amount of concentrated sulfuric acid, and heat and reflux until an ester layer appears. Cool, separate the ester layer, wash with alkali, water, dry, and then fractionate under reduced pressure to obtain the product. The content of industrial product ethyl methacrylate is ≥98%. Raw material consumption quota: methacrylic acid 950kg/t, ethanol 900kg/t.
2. Acetone cyanohydrin reacts with sulfuric acid to form methacrylamide, which is then hydrolyzed and esterified with ethanol to obtain a crude product, which is then dehydrated and distilled to obtain the finished product.
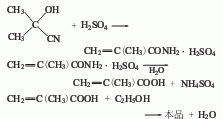
Purpose
1. Commonly used polymerized monomers. It can be used as an intermediate for adhesives, coatings, fiber treatment agents, and molding materials. It can also be used to make acrylic copolymers. It can be copolymerized with methyl methacrylate to improve its brittleness. It is also used to make organic glass, synthetic resin and molding powder.
2. Used to prepare polymers and copolymers, synthetic resins, organic glass and coatings, etc. [24]
extended-reading:https://www.cyclohexylamine.net/potassium-acetate-glycol-solution-polycat-46/
extended-reading:https://www.newtopchem.com/archives/45114
extended-reading:https://www.newtopchem.com/archives/827
extended-reading:https://www.newtopchem.com/archives/44447
extended-reading:https://www.newtopchem.com/archives/623
extended-reading:https://www.morpholine.org/category/morpholine/page/5390/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/9.jpg
extended-reading:https://www.newtopchem.com/archives/44011
extended-reading:https://www.newtopchem.com/archives/44621
extended-reading:https://www.bdmaee.net/dabco-8154-amine-catalyst-dabco-8154-catalyst-dabco-8154/
















Comments