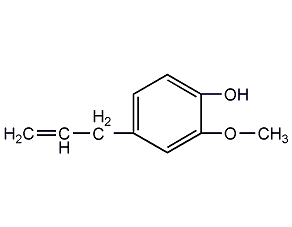
Structural formula
| Business number | 02CA |
|---|---|
| Molecular formula | C10H12O2 |
| Molecular weight | 164.2 |
| label |
eugenol, 4-allyl-2-methoxyphenol, 1-Hydroxy-2-methoxy-4-allylbenzene, 4-allylguaiacol, 4-Allyl-2-methoxyphenol, artificial flavors |
Numbering system
CAS number:97-53-0
MDL number:MFCD00008654
EINECS number:202-589-1
RTECS number:SJ4375000
BRN number:1366759
PubChem number:24862128
Physical property data
1. Properties: Colorless or light yellow liquid, the color deepens when exposed to the air or under strong light. There is a strong clove aroma.
2. Melting point (ºC): -9.2~-9.1
3. Boiling point (ºC): 253.2
4. Relative density (20℃, 4 ℃): 1.0672
5. Relative density (25℃, 4℃): 1.065220
6. Refractive index at room temperature (n20 ): 1.5405
7. Refractive index at room temperature (n25): 1.534530
8. Dissolution Properties: Slightly soluble in water, soluble in ethanol, acetone, ethyl acetate, ether, chloroform and other organic solvents.
9.Flash point (℃): >110
Toxicological data
Toxicity classification Poisoning
Acute toxicity: Oral - Rat LD50: 1930 mg/kg; Oral - Mouse LC50: 3000 mg/kg.
Irritation Data: Skin - Rabbit 100 mg/24 hours Severe.
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 48.72
2. Molar volume (cm3/mol): 156.2
3. Isotonic specific volume (90.2K ): 384.3
4. Surface tension (dyne/cm): 36.5
5. Dielectric constant:
6. Dipole moment (10-24cm3):
7. Polarizability: 19.31
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: 3
6. Topological molecule polar surface area 29.5
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 145
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxides.
2. Colorless to light yellow liquid. Rich caramel sweet aroma. The color turns brown and black after being stored for a long time and exposed to air.
3. Exist in oriental tobacco leaves and smoke.
4. Found in clove oil, cinnamon leaf oil, cinnamon bark oil, camphor oil, nutmeg oil, etc.
Storage method
Brown glass bottle with light-sealed packaging. Store in a cool and dark place.
Synthesis method
1. Eugenol mainly exists in clove basil oil and camphor cinnamon leaf oil. It is a component of various aromatic oils, especially clove oil, cinnamon leaf oil, basil oil, and laurel oil. Although eugenol can also be prepared by synthetic methods, it is generally isolated and extracted from plants or aromatic oils in industry.
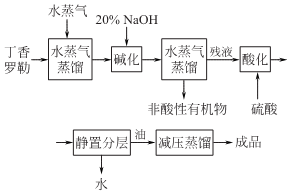
Take the clove basil and remove the fine roots and Foreign matter, dried and chopped, steam distilled. When distilling, maintain a strong fire and return the distillate to the pot to increase the oil yield. When the final steamed oil-water mixture is around 45°C, the relative density of the essential oil is close to water, and the distillate is white and turbid; when the temperature is between 30 and 40°C, part of the oil floats on the water (the relative density of ocimene is about 0.9) , the other part of the oil sinks underwater (the relative density of eugenol is greater than that of water).
Add 20% sodium hydroxide to the oil-water mixture, and then perform steam distillation to remove all non-acidic substances to obtain a eugenol sodium salt solution. Then add 30% sulfuric acid to neutralize it to pH=2~3 (water layer) with stirring at 50°C, let it stand and separate into layers, take off the crude eugenol oil in the lower layer and conduct distillation under reduced pressure to obtain eugenol.
It is produced by the reaction of o-methoxyphenol and allyl bromide, followed by heating and rearrangement.
2.Isolation method: Add excess sodium hydroxide aqueous solution to the natural essential oil containing eugenol, stir thoroughly, and This generates sodium eugenol that is soluble in water. After the oil layer is separated, the alkaline aqueous solution containing sodium eugenol is acidified with dilute sulfuric acid, and the eugenol is re-precipitated. Separate the eugenol oil layer, wash and dry it, and then distill it under reduced pressure to obtain refined eugenol.
3.The synthesis method uses o-methoxyphenol and allyl chloride as raw materials, in the presence of metallic copper, Heating to about 100℃, eugenol can be generated in one step. Since the boiling points of the three isomers are very close, the separation and purification work is very complicated. Therefore, the isolation method to generate eugenol is still the main method at present.
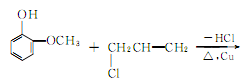
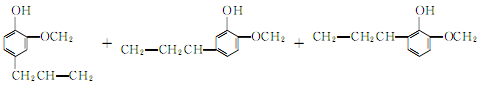
4. Tobacco: OR, 26; can be separated from clove oil. When the synthetic method uses guaiacol as raw material to prepare eugenol, there are many side reactions, and it is difficult to separate and purify.
Purpose
1. In addition to being used in medicine, it can also be used as a raw material for synthetic fragrances. It can also be used to prepare carnation-flavored daily flavors and can also be used as edible spices.
2.Mainly used for preparing mint, nuts, spicy foods and tobacco flavors. The dosage used in meat is 40~2000mg/kg; in gum, 500mg /kg; in condiments, 9.6~100mg/kg; in baked goods, 33mg/kg; 32 in candiesmg/kg; 3.1 in cold drinks mg/kg; 1.4mg/kg in soft drinks; 0.6 in puddings. In addition, eugenol has antiseptic and strong bactericidal properties and is found naturally in many essential oils.
3.Eugenol is the main fragrance agent for preparing clove, carnation, carnation and other flavors. It is used for preparing woody and oriental flavors. Fixatives and modifiers for fragrances. It is also often used in mint, nuts, spicy food flavors and tobacco flavors, and is also an important raw material for the synthesis of vanillin. It is also used in medicine and oral hygiene products. Eugenol is a commonly used analgesic drug in dentistry. It mainly anesthetizes dental nerves, slows down stimulation and plays an analgesic effect.
4. It is used in the blending of cosmetics, soaps, food and other flavors. Used in the ink industry as an anti-drying agent.
5. Also used as insecticide and preservative.
extended-reading:https://www.newtopchem.com/archives/45087
extended-reading:https://www.morpholine.org/nn-dicyclohexylmethylamine/
extended-reading:https://www.newtopchem.com/archives/39514
extended-reading:https://www.newtopchem.com/archives/862
extended-reading:https://www.newtopchem.com/archives/category/products/page/26
extended-reading:https://www.newtopchem.com/archives/1885
extended-reading:https://www.bdmaee.net/cas-3542-36-7/
extended-reading:https://www.morpholine.org/category/morpholine/
extended-reading:https://www.bdmaee.net/pentamethyldipropene-triamine-2/
extended-reading:https://www.newtopchem.com/archives/40413




















Comments