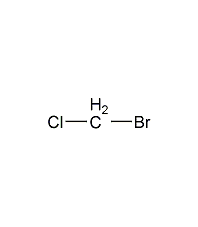
Structural formula
| Business number | 01HS |
|---|---|
| Molecular formula | CH2ClBr |
| Molecular weight | 129.38 |
| label |
chlorobromomethane, Methylene bromide chloride, Chlorobromethylene, methylene bromide chloride, Bromochloro-methane, Chloromethylbromide, Monochloromonobromomethane, Methylene chlorobromide, CB, small fire extinguishing agent, mineral flotation agent, Penetrant for coatings |
Numbering system
CAS number:74-97-5
MDL number:MFCD00000880
EINECS number:200-826-3
RTECS number:PA5250000
BRN number:1730801
PubChem number:24863036
Physical property data
1. Properties: colorless and transparent liquid with a special odor similar to chloroform. [1]
2. Melting point (℃): -88[2]
3. Boiling point (℃): 68.1[3]
4. Relative density (water = 1): 1.93[4]
5. Relative vapor Density (air=1): 4.5[5]
6. Saturated vapor pressure (kPa): 15.3 (20℃)[6]
7. Critical temperature (℃): 297[7]
8. Critical pressure (MPa): 6.08[8]
9. Octanol/water partition coefficient: 1.41[9]
10. Solubility: insoluble in water, soluble in ethanol, acetone, Most organic solvents such as ether, benzene, carbon tetrachloride, chloroform, methanol, etc. [10]
11. Kinematic viscosity (m2/s, 20ºC): 0.3486×10-6
12. Kinematic viscosity (m2/s, 40ºC): 0.2949×10-6
13. Kinematic viscosity ( m2/s, 60ºC): 0.2659×10-6
14. Heat of evaporation (KJ/kg, b.p.): 232.02
15. Heat of formation (KJ/mol, 25ºC): -50
16. Specific heat capacity (J/(kg·K), constant pressure): 0.41
17 .Solubility (%, water, 25ºC): 0.9
18. Liquid phase standard hot melt (J·mol-1·K-1) : 100.8
19. Gas phase standard claimed heat (enthalpy) (kJ·mol-1): -44.8
20. Gas phase standard entropy (J· mol-1·K-1): 287.59
21. Gas phase standard formation free energy (kJ·mol-1): 34.0
22. Gas phase standard hot melt (J·mol-1·K-1): 52.71
Toxicological data
1. Acute toxicity[11] LD50: 5000mg/kg (rat oral); 4300mg/kg (mouse oral Oral)
2. Irritation No data available
3. Subacute and chronic toxicity[12] Rats, rabbits, dogs and other animals are exposed to 5.3g/m3, 7 hours a day, after 14 days,There is no poisoning reaction and no pathological changes.
4. Mutagenicity[13] Microbial mutagenicity: Salmonella typhimurium 10mg/dish. Cytogenetic analysis: hamster lung 1 μmol/L. Sister chromatid exchange: 5μmol/L.
Ecological data
1. Ecotoxicity[14] LC50: 338mg/L (48h) (medaka)
2. Biodegradability[15] MITI-I test, initial concentration 100ppm, sludge concentration 30ppm, degradation 4% after 4 weeks
3. Non-biodegradability[16] In the air, when the hydroxyl radical concentration is 5.00×105 pieces/cm3, the degradation half-life is 145d (theoretical).
4. Other harmful effects[17] The ozone depletion potential (ODP) is 0.12, which will cause damage to the ozone layer .
Molecular structure data
1. Molar refractive index: 19.26
2. Molar volume (cm3/mol): 69.6
3. Isotonic specific volume (90.2K ): 162.5
4. Surface tension (dyne/cm): 29.7
5. Polarizability (10-24cm3): 7.63
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 1.7
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 3
8. Surface charge: 0
9. Complexity: 4.8
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[18] Stable
2. Incompatible substances [19] Strong oxidants, strong bases, alkali metals
3. Polymerization hazards[20] No polymerization
4. Decomposition products [21] Hydrogen chloride, hydrogen bromide
Storage method
Storage Precautions[22] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. Keep container tightly sealed. They should be stored separately from alkali metals and food chemicals, and avoid mixed storage. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
None
Purpose
1. Used as small fire extinguishing agent, mineral flotation agent and penetrant for coatings. [23]
extended-reading:https://www.bdmaee.net/niax-d-50-tertiary-amine-catalyst-momentive/
extended-reading:https://www.cyclohexylamine.net/high-quality-bismuth-octoate-cas-67874-71-9-bismuth-2-ethylhexanoate/
extended-reading:https://www.newtopchem.com/archives/859
extended-reading:https://www.newtopchem.com/archives/39412
extended-reading:https://www.newtopchem.com/archives/472
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/26.jpg
extended-reading:https://www.morpholine.org/polyurethane-metal-carboxylate-catalyst-polycat-46-catalyst-polycat-46/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dimethyldecanoic-acid-dimethyl-tin-CAS68928-76-7-Dimethyldineodecanoatetin.pdf
extended-reading:https://www.morpholine.org/acetic-acid-potassium-salt/
extended-reading:https://www.cyclohexylamine.net/lupragen-n206-tegoamin-bde-pc-cat-np90/




















Comments