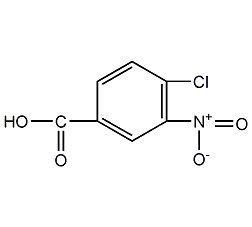
Structural formula
| Business number | 02BR |
|---|---|
| Molecular formula | C7H4ClNO4 |
| Molecular weight | 201.57 |
| label |
3-nitro-4-chlorobenzoic acid |
Numbering system
CAS number:96-99-1
MDL number:MFCD00007079
EINECS number:202-550-9
RTECS number:DG5425050
BRN number:783626
PubChem number:24855387
Physical property data
1. Properties: light yellow crystalline powder.
2. Density (g/mL, 20℃): 1.645
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 181-182
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, kPa): Undetermined
p>
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Not determined
11. Vapor pressure (mmHg, 20.2ºC): Not determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V /V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Easily soluble in hot water and soluble in alcohol.
Toxicological data
1. Acute toxicity: Rat oral LD50: 3150mg/kg; oral LD50 of wild birds: 75mg/kg; 2. Mutagenicity: Mutant microbial test: bacteria - Salmonella typhimurium, 500μg/plate;
Ecological data
None
Molecular structure data
1. Molar refractive index: 44.62
2. Molar volume (cm3/mol): 125.7
3. Isotonic specific volume (90.2K ): 360.7
4. Surface tension (dyne/cm): 67.7
5. Polarizability (10-24cm3): 17.69
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 83.1
7. Number of heavy atoms: 13
8. Surface charge: 0
9. Complexity: 227
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stabilitySex
None
Storage method
None
Synthesis method
It is obtained by nitration of p-chlorobenzoic acid. There are several methods for nitrification operation. (1) Add p-chlorobenzoic acid and sulfuric acid to industrial hydrochloric acid in batches. After the addition is completed, keep the reaction at 50-65°C for 5 hours and leave it overnight. Then pour the nitrate into ice water, filter it dry, and wash it with water to obtain the crude product. Then dissolve it with ethanol, decolorize it, filter it, precipitate crystals in distilled water, and dry it to get the finished product. (2) Nitrate p-chlorobenzoic acid in concentrated nitric acid with a molar excess of 15 times, and react for 6 hours below 20°C. The yield is 90%. (3) Use methylene chloride as the solvent and use mixed acid to perform nitration at its boiling point, with a yield of more than 97%. In addition, this product can also be obtained from p-chlorotrichlorotoluene through the following reaction. The nitrification reaction was carried out at 55-60°C for 15 minutes, and the yield was 92%.
Purpose
Organic synthesis intermediate, used in the production of dyes and pharmaceuticals to manufacture the drug methylimidazole.
extended-reading:https://www.bdmaee.net/nt-cat-dmdee-catalyst-cas110-18-9-newtopchem/
extended-reading:https://www.cyclohexylamine.net/low-odor-polyurethane-catalyst-polyurethane-rigid-foam-catalyst/
extended-reading:https://www.bdmaee.net/niax-ef-350-low-odor-balanced-tertiary-amine-catalyst-momentive/
extended-reading:https://www.newtopchem.com/archives/654
extended-reading:https://www.bdmaee.net/dabco-t-26-catalyst-cas11207-74-9-evonik-germany/
extended-reading:https://www.newtopchem.com/archives/40230
extended-reading:https://www.newtopchem.com/archives/968
extended-reading:https://www.newtopchem.com/archives/44613
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/130-2.jpg
extended-reading:https://www.newtopchem.com/archives/206
















Comments