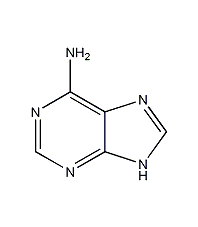
Structural formula
| Business number | 01HC |
|---|---|
| Molecular formula | C5H5N5 |
| Molecular weight | 135 |
| label |
1H-Purine-6-amine, 6-aminourea ring, 6-aminopurine, glandular urine ring, adenoidin, pancreatic acid, Vitamin B4, 6-Aminopurine, 6-Amino-1H-purine, 9H-Purin-6-ylamine, 3,6-Dihydro-6-iminopurine, 6-Aminopurine, Vitamin B4, Plant Growth Regulator, Heterocyclic compounds |
Numbering system
CAS number:73-24-5
MDL number:MFCD00041790
EINECS number:200-796-1
RTECS number:AU6125000
BRN number:5777
PubChem number:24891349
Physical property data
1. Properties: White fine powder crystals with a strong salty taste. The industrial product is slightly yellow crystal
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density (g/mL, air=1) : Uncertain
4. Melting point (ºC): >360 (lit.)
5. Boiling point (ºC, normal pressure): Uncertain
6 . Boiling point (ºC, 5.2 kPa): Uncertain
7. Refractive index: Uncertain
8. Flash point (ºC): 220
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25 ºC): Uncertain
12. Saturated vapor pressure (kPa, 60 ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical Temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16. Log value of oil-water (octanol/water) partition coefficient: Uncertain
15. p>
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (%, V/V): Uncertain
19. Dissolution Properties: Insoluble in cold water (0.5 g/L, 20 ºC), soluble in boiling water, acid and alkali, slightly soluble in ethanol, insoluble in ether and chloroform.
Toxicological data
Rat oral LD50: 745mg/kg
Ecological data
None
Molecular structure data
1. Molar refractive index: 37.03
2. Molar volume (cm3/mol): 83.8
3. Isotonic specific volume (90.2K ): 278.9
4. Surface tension (dyne/cm): 122.7
5. Polarizability (10-24cm3): 14.68
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 8
6. Topological molecule polar surface area 80.5
7. Number of heavy atoms: 10
8. Surface charge: 0
9. Complexity: 127
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
The production workshop should have good ventilation, equipment should be sealed, and operators should wear protective equipment.
Storage method
Stored in a cool, dry and dark place. Packed in iron drums lined with polyethylene plastic bags. Store in a cool, ventilated and dry place. Heat, moisture and sun protection. Store and transport according to regulations on toxic chemicals.
Synthesis method
4,6-Dichloro-5-nitropyrimidine is ammoniated with ammonia to obtain 4,6-diamino-5-nitropyrimidine, which is then cyclized with formic acid, formamide and sodium thiosulfate. . The process is as follows: Dissolve 4,6-dichloro-5-nitropyrimidine in ethanol, heat to 75-78°C and reflux for 0.5h, add ammonia water, and then raise the temperature and reflux for 1.5h. Cool, filter, wash with water and dry to obtain 4,6-diamino-5-nitropyrimidine (amide). Add formamide, water, and ammonia to the reaction tank. Raise the temperature to 100-130℃ and add insurance powder in batches. After the addition is completed, lower the temperature to 100°C, add formic acid, then raise the temperature to 170°C and keep it warm for 2 hours. The solvent was recovered by distillation under reduced pressure. After steaming, add water to dissolve while hot, and add activated carbon to decolorize twice. Adjust the pH to 8-9 with ammonia water, cool, and a light yellow solid will precipitate. Filter and wash with water until there is no sulfate radical. Dry to obtain adenine. Adenine is also recovered as a by-product of the glutamate fermentation process. The industrial product is slightly yellow crystal.
Method 1. Synthesis method using hypoxanthine as raw material
Preparation of 6-chloropurine: 24g hypoxanthine boutique, 80ml dimethylaniline and 300ml phosphorus oxychloride, 150℃ oil bath, reflux for 50 minutes, change the water bath, heat to 70-80°C, and concentrate in vacuum until no more steam can be evaporated. Pour the concentrated solution into 300g ice cubes, stir vigorously until completely dissolved, neutralize with 10mol/L sodium hydroxide solution to pH 11-12, extract with toluene until the toluene layer is colorless, adjust the water with 10mol/L sodium hydroxide in the middle The layer pH is maintained at pH 11-12. Cool, acidify the water layer with concentrated hydrochloric acid to pH 1-2, add 70g of sodium chloride to saturate it, cool it in the refrigerator for 24 hours, filter, rinse the filter residue with a small amount of ice water, dissolve it with 8-10 times hot water, and add 4g of activated carbon for decolorization (Activated carbon is eluted with 200 ml of absolute ethanol, concentrated, and part of the product is recovered), filter while hot, and the filtrate is cooled and crystallized. Then recrystallize with 12 times the amount of distilled water and dry at 100°C to obtain 8.5-9g of 6-chloropurine.
Hypoxanthine [phosphorus oxychloride, dimethylaniline] → [150℃, 5min] 6-chloropurine
Preparation of 6-hydroxylamine purine Dissolve 19.74g of hydroxylamine hydrochloride in absolute ethanol , add 18.69g of potassium hydroxide, heat to reflux for 15 minutes, filter the potassium chloride precipitate generated by cooling and wash with absolute ethanol, combine the filtrate and washing liquid to obtain a neutral clear solution. Add 6-chloropurine, reflux in a water bath for 6 hours and then keep at room temperature overnight. Filter the precipitated 6-hydroxylamine purine, wash with water and absolute ethanol in sequence, and dry in a desiccator to obtain 8.8g of product, mp 245-251°C.
6-Chloropurine [hydroxylamine hydrochloride, potassium hydroxide, absolute ethanol] → [pH7, 6h] 6-hydroxylamine purine
Preparation of 6-aminopurine phosphate 6-hydroxylamine purine 5g, add 80ml water , adjust the pH value to 9 with 10% sodium hydroxide solution, add 20g of industrial insurance powder in batches with stirring at 90°C, and maintain pH 9, stir for 1 hour until the solution is clear, cool, and leave at room temperature overnight, filter, and wash the filter cake with water. , dried at 100°C to obtain 1.75g of 6-aminopurine. Take 4.05g of 6-aminopurine, add 100ml of distilled water and 4.2g of phosphoric acid, heat to dissolve, concentrate, filter, wash with absolute ethanol, and dry in vacuum to obtain 6.3g of 6-aminopurine phosphate.
6-Hydroxyaminopurine [sodium dithionite, sodium hydroxide] → [pH9, 90℃, 1h] 6-aminopurine [phosphoric acid] → 6-aminopurine phosphate
Method 2. Use propylene glycol Synthesis method using diethyl acid as raw material
The preparation ratio (mass ratio) of 4,6-dihydroxypyrimidine is sodium ethoxide:formamide:diethyl malonate=7:2.13:1.
$Sodium ethoxide is first added to the reactor, then formamide is added, heated to 30°C and diethyl malonate is added, heated to 75-80°C to evaporate the fraction, and when drying is fast, replace the vacuum distillation device Concentrate to dryness, add water to dissolve, cool, acidify with hydrochloric acid to pH 4, cool and filter, wash with ice water, and dry to obtain a yellow product.
Diethyl malonate [formamide, sodium ethoxide] → [30℃; 74-81℃, pH4] 4.6-dihydroxypyrimidine
4, 6-dihydroxy-5-nitropyrimidine Preparation: Cool 100ml of nitric acid, add 36ml of concentrated sulfuric acid while stirring, cool to 30°C and add 48g of 4,6-dihydroxypyrimidine in portions., when adding materials, keep the internal temperature at 30-35°C. After the addition, heat the water bath to 37-40°C and stir for 1.5 hours. Pour the reaction solution into 25g of crushed ice, place, filter, wash with ice water until neutral, and filter with suction. Dried light yellow product.
4, 6-dihydroxypyrimidine [nitric acid, sulfuric acid] → [37-40℃, 1.5h] 4, 6-dihydroxy-5-nitropyrimidine
4, 6-dichloro-5 -Preparation of nitropyrimidine 120ml of phosphorus oxychloride, add 60g of 4,6-diamino-5-nitropyrimidine while stirring, heat to 50°C, add dimethylaniline dropwise, reflux for 1 hour after the dripping is completed, and complete the reaction Pour the liquid into 900g of crushed ice and leave it until all the ice cubes are dissolved. Filter and wash with ice water to obtain a light yellow product.
4, 6-dihydroxy-5-nitropyrimidine [phosphorus oxychloride, dimethylaniline] → 4, 6-dichloro-5-nitropyrimidine
4,6-diamino Preparation of -5-nitropyrimidine Dissolve 4,6-dichloro-5-nitropyrimidine in 300ml of ethanol, slowly reflux in a water bath and slowly add 400ml of ammonia water dropwise, keep the temperature at about 65°C during the dropwise addition, and continue to reflux after the addition 1.5h, cool and let stand overnight, then filter, wash with ice water and dry to obtain a brown product. 4, 6-dichloro-5-nitropyrimidine [ethanol, ammonia] → 4,6-diamino-5-nitropyrimidine
Preparation of 6-aminopurine phosphate formamide 200ml, add 4 under stirring , 26g of 6-diamino-5-nitropyrimidine and 30ml of formic acid, raise the temperature to 110°C, add 25g of insurance powder in batches, and control the internal temperature to 110-120°C, after adding, raise the temperature to 180-190°C, reaction 2.5 h, cooled overnight, filtered with suction, washed with ethanol and ice water until neutral, then recrystallized with 600 ml of distilled water, filtered with suction and dried to obtain light yellow 6-aminopurine. Add 250 ml of distilled water, add 0.0125 g of phosphoric acid, adjust the pH to 1-2, heat, decolorize the activated carbon, filter while hot, filter the filtrate after cooling, and dry to obtain the product.
4, 6-diamino-5-nitropyrimidine [methanol, formamide, sodium dithionite] → 6-aminopurine [phosphoric acid] → 6-aminopurine phosphate.
Purpose
1. Used for the production of adenosine, ATP, ADP, new anti-AIDS drugs, vitamin B4 and plant growth hormone 6-benzyladenine, etc.
2. Biochemical research and drug analysis can prevent and treat leukopenia caused by various causes. It is a component of nucleic acids and participates in the synthesis of RNA and DNA in organisms. When white blood cells are lacking, it can promote the proliferation of white blood cells. It is used to prevent and treat leukopenia and acute granulocytopenia. Can also be used for blood storage.
Note: Since this drug is a nucleic acid precursor, when used concurrently with tumor radiotherapy or chemotherapy, the possibility of promoting tumor development should be considered. During injection, it needs to be dissolved in 2 ml of disodium hydrogen phosphate buffer, injected slowly, and cannot be mixed with other drugs for injection.
3. It is an important intermediate in the preparation of adefovir dipivaloyloxymethyl ester.
extended-reading:https://www.newtopchem.com/archives/748
extended-reading:https://www.bdmaee.net/lupragen-n204/
extended-reading:https://www.morpholine.org/category/morpholine/page/5403/
extended-reading:https://www.newtopchem.com/archives/39945
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/102-6.jpg
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/1-3.jpg
extended-reading:https://www.newtopchem.com/archives/855
extended-reading:https://www.bdmaee.net/jeffcat-z-110-catalyst-cas111-42-2-huntsman/
extended-reading:https://www.newtopchem.com/archives/40222
extended-reading:https://www.bdmaee.net/dioctyltin-dilaurate-dotdl/
















Comments