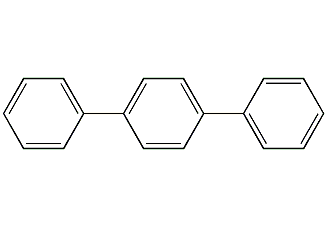
Structural formula
| Business number | 025J |
|---|---|
| Molecular formula | C18H14 |
| Molecular weight | 230.3 |
| label |
To terphenyl, diphenylbenzene, terphenyl, 1,4-diphenylbenzene, 4-phenylbiphenyl, p-diphenylbenzene, p-terphenyl, 4-Phenyldiphenyl, p-Triphenyl, 4-Terphenyl, PTP |
Numbering system
CAS number:92-94-4
MDL number:MFCD00003061
EINECS number:202-205-2
RTECS number:WZ6475000
BRN number:1908447
PubChem number:24869419
Physical property data
1. Character: white scaly crystal
2. Relative density (25℃, 4℃): 0.8790 315.6
3. Relative density (20℃, 4℃): 1.06
6. Boiling point (ºC, 6kPa): 250
7. Standard heat of combustion (enthalpy) of crystalline phase (kJ·mol-1): -9246.7
8. Flash point (ºC): 207
9. Critical temperature (K): 634.85
10. Critical pressure (MPa): 2.99
11. Critical density (g·cm-3): 0.316
12. Critical volume (cm3·mol -1): 729
13. Critical compression factor: 0.289
14. Eccentricity factor: 0.528
15. Gas phase standard Heat of combustion (enthalpy) (kJ·mol-1): -9362.6
16. The gas phase standard claims heat (enthalpy) (kJ·mol-1): 278.7
17. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): 162.8
18. Solubility: soluble It is slightly soluble in ether and carbon disulfide, extremely insoluble in ethanol and acetic acid.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 75.43
2. Molar volume (cm3/mol): 220.0
3. Isotonic specific volume (90.2K ): 553.9
4. Surface tension (dyne/cm): 40.2
5. Dielectric constant (F/m): 2.85
6. Extreme conversion rate (10-24cm3): 29.90
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 18
8. Surface charge: 0
9. Complexity: 198
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be sealed and stored away from light.
Synthesis method
(1) Acetaminophenyl is obtained by nitration, reduction, and acetylation of biphenyl, and then reacts with dinitrogen trioxide to obtain N-nitrosoacetaminobiphenyl, which is then reacted with benzene. This method has a long process route, cumbersome and complicated operations, low yield (only 8%), high cost, low product quality, and serious equipment corrosion.
(2) It is obtained by separating polyphenylene. Among the by-products of biphenyl production, there are p-diphenylbenzene, o-diphenylbenzene, m-diphenylbenzene, m-triphenylbenzene and other biphenyls. According to their different melting points, boiling points and solubility, they can be produced by sublimation and washing methods.
Purpose
Mainly used as a scintillator, used in the production of plastic particles and plastic flakes, or in the preparation of biphenyl liquid crystals One of the basic intermediates of materials, it is also the basic raw material for the synthesis of antibacterial cyclic peptide (4-carboxy-terphenyl, CTP). 4,4-dicarboxyterphenyl (DCTP) can also be prepared, which is the main raw material for preparing biphenyl polyamide materials.
extended-reading:https://www.bdmaee.net/dabco-ne500-catalyst-cas10861-07-1-evonik-germany/
extended-reading:https://www.cyclohexylamine.net/cas-2273-43-0-monobutyltin-oxide-butyltin-oxide/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2016/06/Niax-Catalyst-A-1-MSDS.pdf
extended-reading:https://www.newtopchem.com/archives/44141
extended-reading:https://www.bdmaee.net/dichlorodi-n-octylstannane/
extended-reading:https://www.bdmaee.net/nt-cat-16-catalyst-cas280-57-9-newtopchem/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/4-1.jpg
extended-reading:https://www.newtopchem.com/archives/44219
extended-reading:https://www.cyclohexylamine.net/low-atomization-catalyst-low-atomization-catalyst-9727/
extended-reading:https://www.newtopchem.com/archives/44807
















Comments