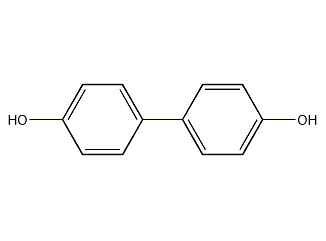
Structural formula
| Business number | 025E |
|---|---|
| Molecular formula | C12H10O2 |
| Molecular weight | 186.21 |
| label |
(1,1'-biphenyl)-4,4'-diol, 4,4'-Diphenol, 4,4'-biphenyldiol, biphenyl, 4,4'-biphenyldiol, (1,1'-biphenyl) -4,4'-diol, 4,4'-phenol, 4,4' - biphenyl diol, Dihydroxybiphenyl, 4,4'- biphenyl phenol, Anti-aging agent |
Numbering system
CAS number:92-88-6
MDL number:MFCD00002348
EINECS number:202-200-5
RTECS number:DV4725000
BRN number:1908886
PubChem number:24863431
Physical property data
1. Properties: White needle-like or flaky crystals.
2. Relative density (g/mL, 25/4℃): 1.22
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 280.5
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor Pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in ethanol and ether, slightly soluble in water and benzene.
Toxicological data
Toxicity classification Highly toxic
Acute toxicity: abdominal cavity - mouse LD50: 100 mg/kg; oral administration - rat LD50: 9850 mg/kg.
Ecological data
Slightly harmful to water.
Molecular structure data
1. Moore's conversionRatio: 54.60
2. Molar volume (cm3/mol): 151.5
3. Isotonic specific volume (90.2K): 410.6
4. Surface tension (dyne/cm): 53.8
5. Polarizability (10-24cm3): 21.64
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: 5
6. Topological molecule polar surface area 40.5
7. Number of heavy atoms: 14
8. Surface charge: 0
9. Complexity: 145
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. White flaky crystals, this product is non-toxic.
2. Easily soluble in ether, ethanol, ethyl acetate, acetone and sodium hydroxide, slightly soluble in benzene and methyl chloride, insoluble in gasoline, carbon tetrachloride and water.
Storage method
This product should be sealed and stored away from light, fireproof, waterproof, and dustproof.
Synthesis method
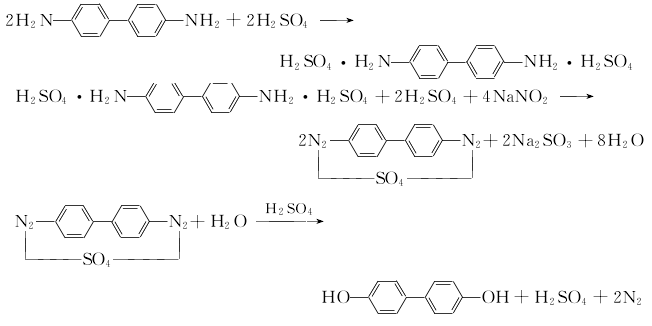
1. Add sulfuric acid to the reaction kettle, raise the temperature to 50°C with stirring, add benzidine, stir and dissolve to form benzidine sulfate, then cool to below 5°C, add sodium nitrite to diazotize the benzidine sulfate , and then under stirring, heat the diazo liquid to boiling to hydrolyze, and precipitate 4,4'-dihydroxybiphenyl. Diazotization takes 20h. It takes 4h for complete hydrolysis. The hydrolyzate is filtered while hot, then washed, dried, and then refined by sublimation to obtain the finished product.

2..Benzidine salt diazotization method: Add sulfuric acid to the reaction kettle, raise the temperature to 50°C with stirring, add amine, stir and dissolve to form benzidine sulfate, then cool to below 5°C, add sodium nitrite, and dissolve the benzidine sulfate. Aniline sulfate is diazotized, and then the diazo liquid is heated to boiling and hydrolyzed under stirring, and 4,4'-dihydroxybiphenyl is precipitated. Diazotization takes 20h. It takes 4h for complete hydrolysis. The hydrolyzate is filtered while hot, then washed, dried, and then refined by sublimation to obtain the finished product. The main reaction is as follows:
① Preparation of sodium 4,4'-dihydroxybiphenyl sulfonate. Heat 30.8g (0.2mol) biphenyl to 80℃, and use a balanced feeder at 80~100℃ in one go Add 54.0 mL of concentrated sulfuric acid with a mass fraction of 98%, raise the temperature to 160°C, hold at a constant temperature for 1 hour, cool to room temperature, and add crushed ice so that the product can be completely dissolved. Prepare 64.0g NaOH into an aqueous solution with a mass fraction of 30%, and add it to the sulfonated product while stirring. A white sulfonate will immediately precipitate; neutralize to neutral (the last 10 mL needs to be added carefully), and cool slightly to 40°C. , filter out the precipitated solid, and dry it to obtain 64.5g of product, with a yield of 90%.
② Preparation of 4,4'-dihydroxybiphenyl: 10.7g (0.03mol) recrystallized sodium 4,4'-biphenyl disulfonate, 13.5 (0.24mol) potassium hydroxide, 31.5g water Mix evenly, use a thermocouple to control the temperature to 340°C, and cool to room temperature naturally after 8 hours of reaction. Add 200 mL of water, heat to dissolve, and filter out insoluble impurities while hot; add dilute hydrochloric acid dropwise to the solution under stirring until the pH value is 4 to 5; filter out the precipitated product, and wash with cold water 2 to 3 times. Dry in the air or vacuum to obtain 4.9 g of powdered product, with a yield of 88%. Colorless flaky crystals were obtained by recrystallization using a solvent of W (ethanol): W (water) = 1:1, with a melting point of 274 to 275°C (sublimation starts from about 220°C).
Purpose
Anti-aging agent DOD is mainly used in rubber and latex. It can prevent aging caused by oxygen and heat, and also has a certain protective effect on harmful metals. It is non-polluting and can be used in light-colored vulcanized rubber products, food packaging rubber and medical latex products. It can also be used in sulfur chloride cold vulcanized products. This product can be used alone with a dosage of 0.4 to 1.5 parts, or it can be used in combination with other antioxidants. The dosage in sulfur chloride cold vulcanized rubber is 0.4 to 0.75 parts.
This product has good heat resistance and is used as a modified monomer for polyester, polyurethane, polycarbonate, polyphenylsulfone and epoxy resin to manufacture excellent engineering plastics and composite materials. As a raw material for liquid crystal polymers, it has a variety of properties and is widely used in many aspects. It can also be spun into high-strength fibers, which can be used to reinforce optical fibers and make composite materials and ropes. This product is used as a dye intermediate and can be used to synthesize photosensitive materials, etc. It is also used as an additive for petroleum products, such as a stabilizer for lubricating greases, etc.
It is an antioxidant for rubber, an important intermediate for heat-resistant engineering plastics, and a dye. Stabilizer, it is also an important precursor for the preparation of alkoxy-substituted liquid crystals. As an anti-aging agent, it is mainly used in rubber and latex. It can prevent aging caused by oxygen and heat, and also has a certain protective effect on harmful metals. It is non-polluting and can be used for light-colored vulcanized rubber products, food packaging rubber and medical latex products. It can also be used for chlorinated sulfur coldIn vulcanized products. It can be used alone with a dosage of 0.4 to 1.5 parts; it can also be used in combination with other antioxidants. Due to its good heat resistance, it can also be used as a modified monomer for polyester, polyurethane, polycarbonate, polyphenylsulfone and epoxy resin to manufacture excellent engineering plastics and composite materials. It can also be spun into high-strength fibers, which can be used to reinforce optical fibers and make composite materials and ropes. Used as dye intermediate, can synthesize photosensitive materials, etc.
extended-reading:https://www.newtopchem.com/archives/44304
extended-reading:https://www.bdmaee.net/2114-2/
extended-reading:https://www.cyclohexylamine.net/thermal-catalyst-polyurethane-delayed-thermal-catalyst/
extended-reading:https://www.bdmaee.net/fascat2001-catalyst-cas301-10-0-stannous-octoate/
extended-reading:https://www.newtopchem.com/archives/44906
extended-reading:https://www.cyclohexylamine.net/polyurethane-gel-type-catalyst-dabco-low-odor-catalyst/
extended-reading:https://www.cyclohexylamine.net/polyurethane-tertiary-amine-catalyst-catalyst-r-8020/
extended-reading:https://www.bdmaee.net/nt-cat-la-200-catalyst-cas10317-48-7-newtopchem/
extended-reading:https://www.newtopchem.com/archives/1902
extended-reading:https://www.cyclohexylamine.net/2-2-dimethylaminoethylmethylamino-ethanol-nnn-trimethylaminoethylethanolamine/
















Comments