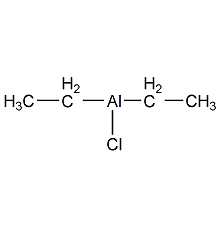
Structural formula
| Business number | 02AE |
|---|---|
| Molecular formula | C4H10AlCl |
| Molecular weight | 120.56 |
| label |
Diethyl aluminum monochloride, diethyl aluminum chloride, Diethylaluminum chloride, Chloro diethyl aluminum, aluminum diethyl monochloride, (C2H5)2AlCl, polymerization catalyst |
Numbering system
CAS number:96-10-6
MDL number:MFCD00000459
EINECS number:202-477-2
RTECS number:BD0558000
BRN number:4123259
PubChem number:24855548
Physical property data
1. Properties: Colorless and transparent liquid.
2. Density (g/mL, 20℃): 0.96
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): -50
5. Boiling point (ºC, normal pressure): 208
6. Boiling point (ºC, 43mmHg): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg, ºC): Undetermined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V) : Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in xylene and gasoline. Soluble in gasoline and aromatic hydrocarbons.
Toxicological data
1. Acute toxicity: Rat inhalation LC50: 11mg/m3;
Ecological data
This substance is slightly hazardous to water.
Molecular structure data
1. Molar refractive index: 43.73
2. Molar volume (cm3/mol): 134.4
3. Isotonic specific volume (90.2K ): 345.0
4. Surface tension (dyne/cm): 43.3
5. Polarizability (10-24cm3): 17.33
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP):
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 2
5. Tautomers.Mass:
6. Topological molecular polar surface area (TPSA): 0
7. Number of heavy atoms: 6
8. Surface charge: 0
9. Complexity: 26.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. The number of uncertain atomic stereocenters: 0
13. The number of determined chemical bond stereocenters: 0
14. The number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with humid air. Avoid contact with alcohols, oxygen, oxidants, acids, alkalis, and water. It is easy to spontaneously ignite when exposed to air and explode when exposed to water.
2. Wash with plenty of water after contact with human body. During the production process, strict protective measures should be taken, production equipment should be sealed to prevent leakage, and operators should wear protective equipment.
Storage method
1. Store in a cool, dry and well-ventilated warehouse. Keep away from fire and heat sources. The storage temperature should not exceed 30℃. Keep container tightly sealed. They should be stored separately from oxidants, acids, alkalis, alcohols, and food chemicals, and avoid mixed storage. It should not be stored in large quantities or for long periods of time. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials. Should be stored under dry nitrogen.
2. This product is prone to violent combustion when exposed to air and reacts violently when exposed to water. Flammable and explosive, must not come into contact with air and water. Packed in steel cylinders, protected by nitrogen. Or dilute it to less than 20%, put it in barrels and protect it with nitrogen seal to prevent leakage. It is safer to use hexane or gasoline to make a 15% to 20% solution.
Synthesis method
1. Aluminum iodine method (sesquimethod) The reaction between ethyl chloride dried by aluminum oxide and aluminum powder in the presence of activator iodine and sesquiethylaluminum chloride produces sesquiethylaluminum chloride. (referred to as sesqui-particle), and then reacted with metallic sodium or sodium chloride to obtain diethyl aluminum chloride.
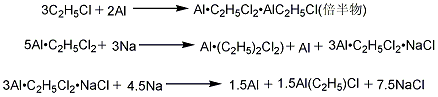
2. Aluminum-magnesium alloy method: Refined ethyl chloride reacts with aluminum-magnesium alloy that has been ball milled and activated by diethyl aluminum chloride, and the finished product is obtained after evaporation.
3. Direct method: Add triethylaluminum and hydrogen to activated aluminum to react to form diethyl aluminum hydride, and then react with ethylene to form triethylaluminum. Triethylaluminum reacts with aluminum trichloride to form diethylaluminum chloride. Raw material consumption (kg/t), aluminum iodine method aluminum powder ≥99.5% 400ethyl chloride ≥99% 1460iodine ≥99.5% 8 metallic sodium ≥99.5% 134.5
Purpose
Catalyst for polyolefin industry, intermediate for manufacturing organic compounds.
This product is mainly used as a polymerization catalyst for polyolefin, butyl rubber and ethylene-propylene rubber and as an intermediate for the synthesis of organometallic compounds and contraceptive pills.
extended-reading:https://www.newtopchem.com/archives/44821
extended-reading:https://www.newtopchem.com/archives/category/products/rigid-foams-catalyst
extended-reading:https://www.newtopchem.com/archives/category/products/page/46
extended-reading:https://www.bdmaee.net/niax-ef-100-low-odor-strong-foaming-catalyst-momentive/
extended-reading:https://www.newtopchem.com/archives/44974
extended-reading:https://www.newtopchem.com/archives/39823
extended-reading:https://www.bdmaee.net/cas-753-73-1/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/115-8.jpg
extended-reading:https://www.newtopchem.com/archives/44066
extended-reading:https://www.newtopchem.com/archives/category/products/page/137




















Comments