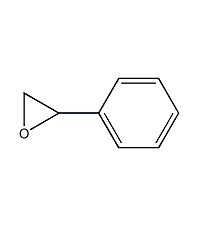
Structural formula
| Business number | 02AD |
|---|---|
| Molecular formula | C8H8O |
| Molecular weight | 120.15 |
| label |
Epoxyphenylene oxide, styrene epoxide, α,β-epoxystyrene, Styrene-7,8-oxide, Phenyloxirane, α,β-Epoxystyrene, 1,2-Epoxystyrene, Epoxy resin thinner, Ether and acetal solvents |
Numbering system
CAS number:96-09-3
MDL number:MFCD00005121
EINECS number:202-476-7
RTECS number:CZ9625000
BRN number:108582
PubChem number:24899628
Physical property data
1. Properties: colorless to light yellow liquid with aromatic smell.
2. Relative density (g/mL, 25/4℃): 1.0469
3. Relative vapor density (g/mL, air=1): 4.14
4. Melting point (ºC): -37
5. Boiling point (ºC, 101.3kPa): 194
6. Boiling point (ºC, 3.33kPa): 91
p>
7. Refractive index (20ºC): 1.535
8. Flash point (ºC): 79
9. Specific rotation (º): Undetermined
7. p>
10. Autoignition point or ignition temperature (ºC): 497.8
11. Vapor pressure (mmHg, 20ºC): <1
12. Saturated vapor pressure ( kPa, 20ºC): 0.048
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15 . Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) distribution coefficient: Undetermined
17. Explosion upper limit (%, V/V ): 22.0
18. Lower explosion limit (%, V/V): 1.1
19. Solubility: insoluble in water, miscible in methanol, ether, tetrachloride Carbon, benzene, acetone, chloroform.
Toxicological data
1. Acute toxicity: rat oral LD50: 2000mg/kg; rabbit transdermal LD50: 2830mg/kg
2. Can be absorbed into the body through inhalation, skin and ingestion. This substance irritates the eyes and skin, causes dizziness, drowsiness, confusion, vomiting, and causes skin allergies. This substance may be a human carcinogen under long-term or repeated exposure.
Ecological data
This substance is harmful to the environment, and special attention should be paid to the pollution of water bodies.
Molecular structure data
1. Molar refractive index: 35.27
2. Molar volume (cm3/mol): 108.4
3.� Isotonic specific volume (90.2K): 278.0
4. Surface tension (dyne/cm): 43.2
5. Dielectric constant:
6. Even Polar distance (10-24cm3):
7. Polarizability: 13.98
Compute chemical data
1. Hydrophobic parameter calculation reference value (XlogP): 1.6
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 1
4. Number of rotatable chemical bonds: 1
5. Number of tautomers:
6. Topological molecular polar surface area (TPSA): 12.5
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 94.7
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 1
13. Determine the number of chemical bond stereocenters Number: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Avoid contact with oxidants, acids, and alkalis. It is flammable and can form explosive mixtures with air.
2. Chemical properties: Under the action of acid, alkali or certain metal salts, the substance may polymerize when heated to 200°C.
3. For its toxicity and protection, please refer to ethylene oxide.
4. Exist in tobacco leaves and smoke.
Storage method
Stored in a cool, ventilated warehouse. Keep away from fire and heat sources. Protect from direct sunlight. Keep container sealed and strictly prohibited from contact with air. They should be stored separately from oxidants, acids, and alkalis, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. The storage area should be equipped with emergency release equipment and suitable containment materials.
Stored in a cool, dry, ventilated warehouse, away from fire and heat sources, moisture-proof, sun-proof, and sealed. Store and transport according to general chemical regulations.
Packed in 200kg galvanized iron drum
Synthesis method
1. Mix 42g peroxybenzoic acid, 30g styrene and 400ml chloroform, and keep at 0℃ for 24h. Take a sample to check and there should be a slight excess of peroxybenzoic acid. Wash the reaction product with excess 10% sodium hydroxide solution to remove benzoic acid. Then wash with water to remove alkali, dry over anhydrous sodium sulfate, collect the 188-192°C fraction by distillation, and obtain 24-26g of styrene oxide.
2. Epoxyphenylene oxide is obtained from styrene, sodium bromide, sulfuric acid and liquid caustic soda through halogenation reaction, saponification reaction and distillation.

Purpose
Used as pharmaceutical and spice intermediates. It is used as an intermediate in the production of benzene glycol and its derivatives, and also as a diluent in the epoxy resin industry.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2019/10/1-4.jpg
extended-reading:https://www.cyclohexylamine.net/polycat-9-trisdimethylaminopropylamine/
extended-reading:https://www.newtopchem.com/archives/39970
extended-reading:https://www.bdmaee.net/butyltintrichloridemincolorlessliq/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-RP208-high-efficiency-reaction-type-equilibrium-catalyst-reaction-type-equilibrium-catalyst.pdf
extended-reading:https://www.newtopchem.com/archives/1103
extended-reading:https://www.newtopchem.com/archives/39605
extended-reading:https://www.newtopchem.com/archives/category/products/page/102
extended-reading:https://www.newtopchem.com/archives/44031
extended-reading:https://www.newtopchem.com/archives/category/products/page/64
















Comments