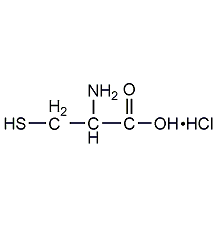
Structural formula
| Business number | 015R |
|---|---|
| Molecular formula | C3H8ClNO2S |
| Molecular weight | 157.62 |
| label |
L-cysteine hydrochloride, L-cysteine hydrochloride hydrate, Alpha-amino-beta-mercaptopropionic acid hydrochloride, L-cysteine hydrochloride, fu amino acid, L-mercaptoalanine hydrochloride anhydrous, L-Cysteine hydrochloride, Cysteine HCl, 2-Amino-3-mercaptopropanoic acid, 3-Mercaptoalanine, Biochemical reagents |
Numbering system
CAS number:52-89-1
MDL number:MFCD00064553
EINECS number:200-157-7
RTECS number:HA2275000
BRN number:3560277
PubChem number:24892395
Physical property data
1. Character: White crystal. Hygroscopic.
2. Density (g/mL, 25/4℃): Not determined
3. Relative vapor density (g/mL, air=1): Not determined Determined
4. Melting point (ºC): 175~178℃ (decomposition)
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 13.33kpa):
7. Refractive index: Not determined
8. Flash point (ºC): Not determined
9. Specific rotation Degree (º,): 5.0° (5mol/L, in hydrochloric acid)
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (kPa , 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient : Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Solubility: Soluble in water, ethanol and acetone. Aqueous solutions are acidic.
Toxicological data
1. Acute toxicity: mouse abdominal LC50: 1250 mg/kg; mouse intravenous LC50: 771 mg/kg; mouse LC50: 3 mg/kg; 2. Mutagenicity: mutation microorganismsTEST system: bacteria - Salmonella typhimurium: 20mg/plate; Cytogenetic analysisTEST system: rodents�-Hamster fibroblasts: 2mg/L
Ecological data
None yet
Molecular structure data
1. Molar refractive index: 28.90
2. Molar volume (cm3/mol): 90.7
3. Isotonic specific volume (90.2K ): 251.5
4. Surface tension (dyne/cm): 58.9
5. Polarizability (10-24cm3): 11.45
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 4
3. Number of hydrogen bond acceptors: 4
4. Number of rotatable chemical bonds: 2
5. Number of tautomers: none
6. Topological molecule polar surface area 64.3
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 75.3
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 1
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 2
Properties and stability
Hygroscopic. Oxidizes and decomposes slowly in air. Aqueous solutions are acidic.
Storage method
Should be sealed and stored in a cool, dry place away from light.
Synthesis method
1. Dissolve cystine in dilute hydrochloric acid, filter and add tin particles to heat and reflux. Dilute the reducing solution with water, remove the remaining tin particles, saturate with hydrogen sulfide, filter, wash the filter residue with a small amount of water, combine the washing liquid and filtrate, concentrate under reduced pressure, cool and crystallize, and dry to obtain L-cysteine hydrochloride.
2. Hydrolyzed by α-keratin contained in hair Cystine can be obtained, and then reduced to cysteine through chemical reduction or electrolysis, and hydrochloric acid is added to form a salt.

Purpose
1. Used for biochemical research. Determination of calcium and magnesium in steel raw materials. Reducing agent for determination of hemolysin. Growth culture and enumeration of anaerobic bacteria.
2.Used in biochemistry Research. Determination of calcium and magnesium in steel raw materials. Reducing agent for determination of hemolysin. It is used to treat acrylonitrile and aromatic poisoning, prevent radiation damage, treat bronchitis and reduce phlegm. It is also used in cosmetics to prevent aging, and as a food additive to promote fermentation and maintain umami taste.
3.Used as analytical reagents, As a masking agent, it is used to measure calcium and magnesium. It can be used as a reducing agent for biochemical research, such as hemolysin determination. Also used for the cultivation of anaerobic bacteria.
extended-reading:https://www.bdmaee.net/niax-b-9-balanced-tertiary-amine-catalyst-momentive/
extended-reading:https://www.cyclohexylamine.net/n-methyl-methylcyclohexylamine/
extended-reading:https://www.newtopchem.com/archives/45105
extended-reading:https://www.morpholine.org/category/morpholine/n-methylmorpholine/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Dioctyl-tin-oxide-CAS870-08-6-FASCAT-8201-catalyst.pdf
extended-reading:https://www.newtopchem.com/archives/44797
extended-reading:https://www.bdmaee.net/fascat9201-catalyst-dibutyl-tin-oxide-fascat9201/
extended-reading:https://www.bdmaee.net/fascat-9102-catalyst/
extended-reading:https://www.newtopchem.com/archives/39611
extended-reading:https://www.cyclohexylamine.net/bis2dimethylaminoethylether-22%e2%80%b2-oxybisnn-dimethylethylamine/




















Comments