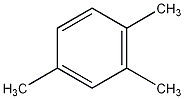
Structural formula
| Business number | 029F |
|---|---|
| Molecular formula | C9H12 |
| Molecular weight | 120.19 |
| label |
pseudoanisolein, Pseudocumol, Pseudocumene, Asymmetrical trimethylbenzene, For the synthesis of spices and dyes, gas liquid chromatography reference materials, Hydrocarbon solvent |
Numbering system
CAS number:95-63-6
MDL number:MFCD00008527
EINECS number:202-436-9
RTECS number:DC3325000
BRN number:1903005
PubChem number:24900445
Physical property data
1. Properties: colorless liquid with aromatic smell. [1]
2. Melting point (ºC): -43.8[2]
3. Boiling point (ºC): 168.9[3]
4. Relative density (water = 1): 0.88[4]
5. Relative vapor Density (air=1): 4.1[5]
6. Saturated vapor pressure (kPa): 1.33 (51.6ºC)[6]
7. Heat of combustion (kJ/mol): -5190.3[7]
8. Critical temperature (ºC): 376.13[8]
9. Critical pressure (MPa): 3.23[9]
10. Octanol/water partition coefficient: 3.8 [10]
11. Flash point (ºC): 44 (CC) [11]
12. Ignition temperature (ºC) ): 500[12]
13. Explosion upper limit (%): 6.4[13]
14. Explosion lower limit (%): 0.9[14]
15. Solubility: insoluble in water, miscible in acetone, petroleum ether, soluble in ethanol, ether, benzene and other organic substances Solvent. [15]
16. Viscosity (mPa·s, 20ºC): 1.01
17. Specific heat capacity (KJ/(kg·K)): 1.7734
18. Thermal conductivity (W/(m·K)): 0.1344
19. Eccentricity factor: 0.379
20. Solubility parameter (J ·cm-3)0.5: 17.945
21. van der Waals area (cm2·mol -1): 1.026×1010
22. van der Waals volume (cm3·mol-1): 81.810
23. Gas phase standard combustion heat (enthalpy) (kJ·mol-1): -5242.72
24. Gas phase standard Claimed heat (enthalpy) (kJ·mol-1): -13.85
25. Gas phase standard entropy (J·mol-1·K-1): 395.31
26. Gas phase standard free energy of formation (kJ·mol-1): 117.5
27. Gas phase standard hot melt (J·mol-1·K-1): 149.71
28. Liquid phase standard combustion heat (enthalpy) (kJ ·mol-1): -5194.77
29. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): -61.80
30. Liquid phase standard entropy (J·mol-1·K-1): 283.38
31. Liquid phase Standard free energy of formation (kJ·mol-1): 105.96
32. Liquid phase standard hot melt (J·mol-1·K-1):212.1
Toxicological data
1. Acute toxicity[16] LC50: 18000mg/m3 (rat inhalation, 4h)
2. Irritation No data available
3. Subacute and chronic toxicity[17] Rabbits were injected subcutaneously with 2~3g/(kg·d), which caused local exudation and necrosis; after 3 weeks, there were cytopenias and temporary leukopenia or increase.
Ecological data
1. Ecotoxicity[18] LC50: 7.72mg/L (96h) (fathead minnow, dynamic) 18mg/L (48h) (medaka)
2. Biodegradability[19]
Aerobic biodegradation (h): 168~672
Anaerobic biodegradation (h): 672~2688
3. Non-biodegradability[20]
Photooxidation half-life in water (h): 1056~43000
Photooxidation half-life in air (h): 1.6~16
4. Bioaccumulation [21] BCF: 33~275 (carp, contact concentration 0.2ppm, contact time 8 weeks); 31~207 (carp, contact concentration 0.02ppm, contact time 8 weeks)
Molecular structure data
1. Molar refractive index: 40.72
2. Molar volume (cm3/mol): 138.2
3. Isotonic specific volume (90.2K ): 320.2
4. Surface tension (dyne/cm): 28.7
5. Polarizability (10-24cm3): 16.14
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 86
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[22] Stable
2. Incompatible substances[23] Strong oxidants, acids, halogens, etc.
3. Polymerization hazard[24] No polymerization
Storage method
Storage Precautions[25] Store in a cool, ventilated warehouse. The storage temperature should not exceed 37°C and should be kept away from fire and heat sources. Keep container tightly sealed. should be kept away from oxidizer, do not store together. Use explosion-proof lighting and ventilation facilities. It is prohibited to use mechanical equipment and tools that are prone to sparks. The storage area should be equipped with emergency release equipment and suitable containment materials.
Synthesis method
C9-C10 aromatics obtained from catalytic reforming or naphtha cracking all contain mixed trimethylbenzenes, such as 1,2,4-trimethylbenzene. Taking reformed aromatics as an example, the 1,2,4-trimethylbenzene content is as high as more than 40%. Products with a purity of more than 99% can be obtained by distillation. For example, two float valve towers (200 layers in total) are used to separate 1,2,4-trimethylbenzene from reformed aromatics, with a purity of 95-97% and a yield of 58- 78%.
Purpose
Used in organic synthesis and pharmaceutical industry, and also used as analytical reagents. [26]
extended-reading:https://www.newtopchem.com/archives/44206
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/16.jpg
extended-reading:https://www.newtopchem.com/archives/44701
extended-reading:https://www.cyclohexylamine.net/polyurethane-catalyst-pc41-hard-foam-catalyst-pc41/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2021/05/139-4.jpg
extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/68.jpg
extended-reading:https://www.bdmaee.net/nt-cat-bdmaee/
extended-reading:https://www.newtopchem.com/archives/category/products/page/51
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/Trisdimethylaminopropylamine--9-PC-CAT-NP109.pdf
extended-reading:https://www.newtopchem.com/archives/40334
















Comments