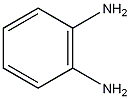
Structural formula
| Business number | 029B |
|---|---|
| Molecular formula | C6H8N2 |
| Molecular weight | 108 |
| label |
1,2-phenylenediamine, O-phenylenediamine, 1,2-diaminobenzene, OPD, Aromatic nitrogen-containing compounds and their derivatives |
Numbering system
CAS number:95-54-5
MDL number:MFCD00007721
EINECS number:202-430-6
RTECS number:SS7875000
BRN number:606074
PubChem number:24856671
Physical property data
1. Properties: colorless monoclinic crystal [6]
2. pH value: 8.7 (1% solution) [7]
3. Melting point (℃): 102~104[8]
4. Boiling point (℃): 252~258[9]
5. Relative density (water=1): 1.03[10]
6. Relative vapor density (air=1): 3.7[11]
7. Saturated vapor pressure (kPa): 0.33 (100℃)[12]
8 .Critical pressure (MPa): 5.18[13]
9. Octanol/water partition coefficient: 0.15[14]
10. Flash point (℃): 156 (CC) [15]
11. Explosion limit (%): 9.8[16]
12. Lower explosion limit (%): 1.5[17]
13. Solubility: slightly soluble in cold water, easily soluble in ethanol, ether, Chloroform. [18]
Toxicological data
1. Acute toxicity[19] LD50: 1070mg/kg (rat oral)
2. Irritation No information available
3. Mutagenicity[20] Microorganisms Mutagenicity: Salmonella typhimurium 1g/L. Micronucleus test: mice take 216mg/kg orally (24h). DNA damage: human lymphocytes 15mmol/L. DNA inhibition: human HeLa cells 7mmol/L. Cytogenetic analysis: human lymphocytes 10mmol/L.
Ecological data
1. It is extremely harmful to water and toxic to fish. Do not let the product enter the water body.
2. Ecotoxicity[21] LC50: 24mg/L (96h) (zebrafish); 280mg/ L (96h) (bluegill sunfish)
3. Biodegradability No data available
4. Non-biodegradability[22] In the air, when the concentration of hydroxyl radicals is 5.00×105/cm3 When, the degradation half-life is 2.2h (theoretical).
Molecular structure data
1. Molar refractive index: 34.72
2. Molar volume (cm3/mol): 93.9
3. Isotonic specific volume (90.2K ): 258.9
4. Surface tension (dyne/cm): 57.5
5. Polarizability (10-24cm3): 13.76
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 2
4. Number of rotatable chemical bonds�:0
5. Number of tautomers: None
6. Topological molecule polar surface area 52
7. Number of heavy atoms: 8
8. Surface charge: 0
9. Complexity: 62.9
10. Number of isotope atoms: 0
11. Determine the atoms Number of stereocenters: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain number of stereocenters of chemical bonds: 0
15. Number of covalent bond units: 1
Properties and stability
1. Stability[23] Stable
2. Incompatible substances[24] Strong acids, strong oxidants, acid anhydrides, acid chlorides
3. Conditions to avoid contact[25] Heat and light
4. Polymerization hazard[26] No polymerization
5. Decomposition products[27] Ammonia
Storage method
Storage Precautions[28] Store in a cool, ventilated warehouse. Keep away from fire and heat sources. The packaging is sealed. They should be stored separately from oxidants, acids, and food chemicals, and avoid mixed storage. Equipped with the appropriate variety and quantity of fire equipment. Suitable materials should be available in the storage area to contain spills.
Synthesis method
1. Use o-nitroaniline as raw material and use sodium sulfide reduction method or catalytic hydrogenation reduction method to prepare o-phenylenediamine. Add 21% sodium sulfide solution and o-nitroaniline to the reduction pot, raise the temperature to 90°C, seal and raise the temperature to 105-110°C, increase the pressure to 0.1-0.2MPa, stir and reduce for 5 hours, cool down to below 40°C, and filter . Heat and melt the filter cake, distill under reduced pressure, and collect the 140-210°C (7.89kPa) fraction which is o-phenylenediamine, with a yield of 70-80%. The hydrogenation process is carried out in an autoclave with a reaction temperature of 95-105°C and a pressure of about 2MPa. The hydrogenation reaction is carried out under the action of a nickel catalyst for about 1 hour until the hydrogen pressure does not drop. The yield of o-phenylenediamine is above 97%.
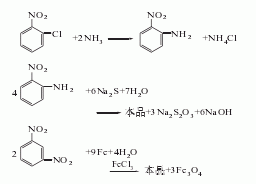
2. The preparation methods are as follows Several kinds.
O-nitroaniline and sodium sulfide reduction method
Use o-nitroaniline and sodium sulfide solution to react at a pressure of 98~196kPa and a reaction temperature of 105~110℃, or at a reaction temperature of 120~130 ℃ and normal pressure, the reaction time is 3 to 4 hours, and o-phenylenediamine is obtained.
O-nitroaniline hydrogenation reduction method
Using o-nitroaniline as raw material and Raney nickel as catalyst, control the temperature in the autoclave to 95~105°C, and continuously vent hydrogen until the pressure does not drop. The product was obtained by stirring the reaction under a pressure of 1.960 MPa.
Among the above two preparation methods, the sodium sulfide reduction method produces a product with higher purity and a relatively mature process route. Many domestic factories use this method to produce o-phenylenediamine. However, this method has poor labor protection conditions and produces more waste water. However, there are still some problems in the process of catalytic hydrogenation, and industrialization needs to be further improved. In addition, there is also the o-dichlorophenyl ammonialysis method, which has been studied by many domestic units, but has not yet been industrialized.
3. Put 69 grams (0.5 mol) o-nitroaniline, 40 ml of 20% sodium hydroxide solution and 200 ml of 95% ethanol. Stir the mixture vigorously and heat on a steam bath until the solution slowly boils. Stop heating, divide 130 grams (2 grams atoms) of needle powder (purity not less than 80%, the dosage is calculated based on 130 grams of material with a purity of 100%) into 10 grams each and add them gradually, the interval J is to keep the solution boiling. . After adding zinc powder, return the mixture and continue stirring for 1 hour. The color of the solution changes from deep red to nearly colorless. The hot mixture was suction filtered, and the remaining zinc was returned to the flask, and extracted twice with 1 ml of hot ethanol in each portion. Add 2 to 3 grams of sodium bisulfite to the combined filtrate, and then concentrate the solution on a steam bath under reduced pressure (with a water pump) to a volume of 125 to 150 ml. After sufficient cooling in an ice-salt bath, collect the light yellow crystals, wash them once with a small amount of ice water, and dry them in a vacuum desiccator. The yield of melted crude o-phenylenediamine in }'7-1 }} is 46 to 50 grams (85-93%). If a purer product is required, the product can be dissolved in 150-175 ml of hot water containing 1 to 2 grams of sodium bisulfite and treated with activated carbon. After sufficient cooling in ice-salt refrigerant, filter out the colorless crystals and wash with 10 = 15 ml of ice water.
The free diamine can also be converted into dihydrochloride by the following method and refined. Dissolve crude o-phenylenediamine in a mixture of 90-100 ml concentrated hydrochloric acid (specific gravity 1.19) and 50-60 ml water containing 2-3 g stannous chloride, and treat the hot solution with activated carbon. In the heat Add 150 ml of concentrated hydrochloric acid to the colorless filtrate, and then cool it thoroughly in an ice-salt bath. Filter out the colorless crystals with suction, wash with a small amount of cold concentrated hydrochloric acid, and Drying over solid sodium hydroxide in vacuum. The yield of o-phenylenediamine dihydrochloride is 77-81 g (85-90% of the theoretical yield based on the weight of o-nitroaniline used
Purpose
1. As a pesticide intermediate, dye intermediate. Used to prepare reductionRaw materials and cationic dyes, used to produce fur yellow brown M, cationic dyes, vat red GG, vat orange GR. Also used in the manufacture of polyamide, polyurethane and leveling agents. It is used in the pesticide industry to synthesize systemic fungicides (carbendazim, thiophanate and thiophanate-methyl).
2. Synthesize a variety of heterocyclic intermediates. Used to prepare antioxidants.
3. O-phenylenediamine is the main intermediate for pesticides, medicines, dyes, and developing rubber agents. O-phenylenediamine and its derivatives can be used as starting materials to react with carbonyl compounds through thermal reaction and microwave irradiation to form benzopolynitrogen heterocyclic aromatic compounds, such as benzimidazoles, quinoxalines and benzazepines. Miscellaneous categories, etc.
When o-phenylenediamine reacts with 2-pyridinecarboxaldehyde, the N4 Schiff base ligand is obtained1[1], but when 2 When -pyridine carboxaldehyde was replaced with 2-acetylpyridine, using methyl chloroformate as the catalyst, the corresponding N4 Schiff base ligand2 was not obtained in the reaction in isopropanol, but benzene was obtained. The six-membered ring product is 2-(2′-pyridyl)quinoxaline3[2].

O-phenylenediamine and two equivalents When the aromatic aldehyde reacts in methanol, the corresponding five-membered nitrogen heterocyclic benzimidazole derivative (formula 1)[3] is generated.

Utilizing o-phenylenediamine and its The derivative and 2-acetylpyridine were used as raw materials, using p-toluenesulfonic acid as the catalyst, in methanol solvent, heated (50~60 ºC) and stirred for 10~20 hours to obtain a benzo six-membered ring product (Formula 2) [4,5].

The raw material aromatic aldehyde in formula 1 When replaced by an acetyl aromatic compound, a benzo seven-membered ring product, that is, a benzazepine compound (Formula 3) is obtained.

Change the reaction method to microwave radiation Benzazepine compounds (Formula 4) can also be obtained by combining o-phenylenediamine and its derivatives with acetyl aromatic compounds.

4. As a pesticide intermediate, Dye intermediates. [29]
extended-reading:https://www.newtopchem.com/archives/40287
extended-reading:https://www.cyclohexylamine.net/low-odor-amine-catalyst-bx405-low-odor-strong-gel-catalyst-bx405/
extended-reading:https://www.cyclohexylamine.net/high-quality-n-methylmorpholine-cas-109-02-4/
extended-reading:https://www.newtopchem.com/archives/43920
extended-reading:https://www.newtopchem.com/archives/593
extended-reading:https://www.newtopchem.com/archives/759
extended-reading:https://www.newtopchem.com/archives/1785
extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/26.jpg
extended-reading:https://www.newtopchem.com/archives/40024
extended-reading:https://www.newtopchem.com/archives/40049
















Comments