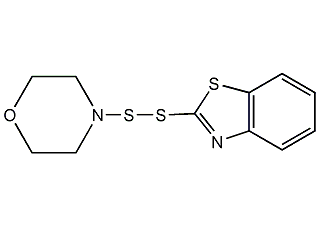
Structural formula
| Physical competition number | 028Z |
|---|---|
| Molecular formula | C11H12N2OS3 |
| Molecular weight | 284.35 |
| label |
accelerator MDB, Accelerator DS, Accelerator MDB, promoter DS, Vulcanizing agent |
Numbering system
CAS number:95-32-9
MDL number:MFCD00059033
EINECS number:202-410-7
RTECS number:DL5953000
BRN number:None
PubChem ID:None
Physical property data
1. Properties: Industrial product is light yellow powder
2. Relative density (g/mL, 20℃): 1.51
3. Relative vapor density (g/mL , air=1): Not determined
4. Melting point (ºC): 123-135
5. Boiling point (ºC, normal pressure): Not determined
6. Boiling point (ºC, KPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition temperature (ºC): Undetermined
11. Vapor pressure (mmHg,ºC): Undetermined Determined
12. Saturated vapor pressure (kPa, ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical Temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Log value of oil-water (octanol/water) partition coefficient: Undetermined
p>
17. Explosion upper limit (%, V/V): Undetermined
18. Explosion lower limit (%, V/V): Undetermined
19. Dissolution Properties: Soluble in chloroform, slightly soluble in carbon disulfide and acetone, insoluble in benzene, petroleum ether, ethanol and water.
Toxicological data
Acute toxicity: Mouse oral LD50: 3mg/kg
Ecological data
Slightly harmful to water bodies.
Molecular structure data
1. Molar refractive index: 78.38
2. Molar volume (cm3/mol): 194.7
3. Isotonic specific volume (90.2K ): 571.2
4. Surface tension (dyne/cm): 74.0
5. Dielectric constant (F/m):
6. Dipole Distance (D):
7, Polarizability (10-24cm3): 31.07
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): 3.1
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 6
4. Number of rotatable chemical bonds: 3
5. Number of tautomers: none
6. Topological molecule polar surface area 104
7. Number of heavy atoms: 17
8. Surface charge: 0
9. Complexity: 251
10. Number of isotope atoms: 0
11. Determine the atomic stereocenter Quantity: 0
12. Uncertain number of stereocenters of atoms: 0
13. Determined number of stereocenters of chemical bonds: 0
14. Uncertain chemical bonds Number of stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. Soluble in chloroform, slightly soluble in carbon disulfide and acetone, insoluble in benzene, petroleum ether, ethanol and water.
2. This product is non-toxic.
Storage method
Store at low temperature and dry place
Synthesis method
1. The accelerator MDB is prepared by reacting the accelerator M with morpholine and sulfur monochloride;

2. Heat 36g accelerator M, 36g morpholine, 7g sulfur and 100ml isopropyl alcohol to 65-70°C, then slowly heat the mixture Slowly treat with 120ml of 1.5mol sodium hypochlorite, and then stir at 55-65°C for 0.5h. After washing, the product is added to the accelerator MDB.
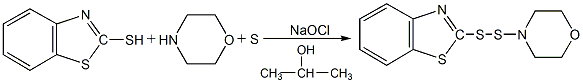
3. Add 100ml ethanol solution ( (containing 20.5g of dimorpholine monosulfide) was mixed and reacted with another 100ml ethanol solution containing 17g of accelerator M. After 5 hours, the reactant was cooled and filtered to obtain the accelerator MDB.
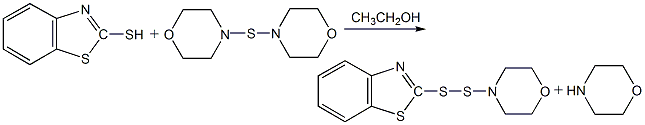
4. Add the accelerator NOBS26g, 3.2g of sulfur, 150ml of ethanol and 8.5g of morpholine were refluxed together for 2 hours. After the reactants were cooled and filtered, the accelerator MDB was obtained.

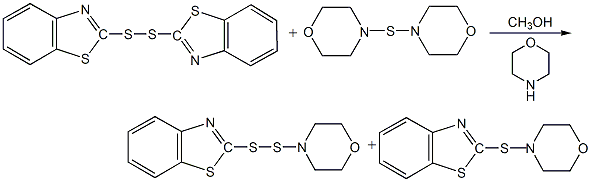
5. Combine morpholine monosulfide , accelerator DM, refluxed with morpholine and methanol. After 1 hour, the reactant was cooled and filtered to obtain MDB.
Purpose
This product is used as a post-effect vulcanization accelerator for rubber and can also be used as a vulcanizing agent. When used as an accelerator, its performance in natural rubber is similar to that of the accelerator CZ (N-cyclohexyl-2-benzothiazole sulfenamide), but the delay is slightly greater. When used in 54.1 (W type) chloroprene rubber, it is equipped with the accelerator PZ (zinc dimethyldithiocarbamate), which can speed up the vulcanization speed, has good scorch performance, and has excellent physical properties of the product. When used in styrene-butadiene rubber, the scorch performance is poorer than the accelerator CZ. It can be combined with stearic acid to promote scorch and improve the tensile strength. When used as vulcanizing agent, it is advisable to add a small amount of thiuram or dithiocarbamate accelerator, which can increase the vulcanization speed and improve the aging resistance of the product. This product is easily dispersed in rubber and has almost no pollution. Mainly used in the manufacture of tires, inner tubes, rubber shoes, sponges, industrial products, etc. When used as a vulcanizing agent, the dosage is 2.5 to 5 parts, combined with about 1 part of accelerator PZ. When used as an accelerator, the general dosage is 0.4 to 1.5 parts.
extended-reading:https://www.bdmaee.net/dioctyltin-dilaurate/
extended-reading:https://www.bdmaee.net/dibutyltin-dibenzoate/
extended-reading:https://www.bdmaee.net/nn-dimethyl-ethanolamine-4/
extended-reading:https://www.morpholine.org/pc41/
extended-reading:https://www.morpholine.org/category/morpholine/4-formylmorpholine/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2020/06/80-2.jpg
extended-reading:https://www.newtopchem.com/archives/846
extended-reading:https://www.bdmaee.net/cyclohexylamine-series-products-2/
extended-reading:https://www.bdmaee.net/nt-cat-tea-catalyst-cas280-57-9-newtopchem/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-33-LX--33-LX-catalyst-tertiary-amine-catalyst-33-LX.pdf
















Comments