
Structural formula
| Business number | 024T |
|---|---|
| Molecular formula | C12H10 |
| Molecular weight | 154.21 |
| label |
phenylbenzene, biphenyl, 1,1'-biphenyl, Bibenzene,Biphenyl, Phenylbenzene, fungicides, aromatic compounds |
Numbering system
CAS number:92-52-4
MDL number:MFCD00003054
EINECS number:202-163-5
RTECS number:DU8050000
BRN number:1634058
PubChem number:24861956
Physical property data
1. Characteristics: White to light yellow flaky crystals, with a sharp odor and a rose-like aroma after dilution.
2. Boiling point (ºC, 101.3kPa): 255.2
3. Melting point (ºC): 69
4. Relative density (g/mL, 20 /4ºC): 1.04
5. Refractive index (n77D): 1.588
6. Kinematic viscosity (m2/s, 100ºC): 0.98×10-6
7. Flash point (ºC, closed): 113
8. Ignition point (ºC): 540
9. Heat of evaporation (KJ/kg, 200ºC): 343.3
10. Heat of fusion (KJ/ mol, 70.5ºC): 18.59
11. Heat of formation (KJ/mol, solid): 96.67
12. Heat of combustion (KJ/mol): 6252.4
13. Specific heat capacity (KJ/(kg·K), constant pressure): 1.61
14. Critical temperature (ºC): 515.7
15. Critical pressure (MPa): 3.38
16. Thermal conductivity (W/(m·K), 100ºC): 1339.77
17. Lower explosion limit (%, V/V): 0.6
18. Explosion upper limit (%, V/V): 5.8
19. Solubility: Insoluble in water, soluble in ether, ethanol, carbon tetrachloride, dioxane, aromatic hydrocarbons, etc. . Dissolves 9.1% in ethanol at 19.5°C.
20. Critical pressure (MPa): 3.38
21. Critical density (g·cm-3): 0.310
22. Critical volume (cm3·mol-1): 497
23. Critical compression factor: 0.262
24 . Eccentricity factor: 0.366
25. Solubility parameter (J·cm-3)0.5: 19.383
26. van der Waals area (cm2·mol-1): 1.066×1010
27. van der Waals Volume (cm3·mol-1): 91.680
28. Gas phase standard combustion heat (enthalpy) (kJ·mol-1 ):-6332.7
29. Gas phase��Accurate claimed heat (enthalpy) (kJ·mol-1): 181.4
30. Gas phase standard entropy (J·mol-1· K-1): 393.78
31. Gas phase standard free energy of formation (kJ·mol-1): 280.1
32. Gas phase standard hot melt (J·mol-1·K-1): 165.28
33. Liquid phase standard heat of combustion (enthalpy) (kJ·mol-1): -6932.3
34. Liquid phase standard claims heat (enthalpy) (kJ·mol-1): 116.0
35. Liquid phase standard entropy (J·mol-1·K-1): 250.2
36. Liquid Phase standard formation free energy (kJ·mol-1): 255.4
37. Crystalline phase standard combustion heat (enthalpy) (kJ·mol-1 sup>): -6250.7
38. Crystal phase standard claims heat (enthalpy) (kJ·mol-1): 99.4
39. Crystal phase Standard entropy (J·mol-1·K-1): 205.9
40. Crystal phase standard formation free energy (kJ·mol-1): 252.0
41. Crystal phase standard hot melt (J·mol-1·K-1): 195
Toxicological data
It is of low toxicity and irritating to humans. Its vapor can irritate the eyes, nose, and trachea, causing loss of appetite, vomiting, etc., and is toxic to the nervous system, digestive system, and kidneys. The oral LD50 in rats is 3.28g/kg. The maximum allowable concentration in the workplace is >1 mg/m3 (coexisting with diphenyl ether). It has a stimulating effect, damages the heart, liver and kidneys, and has toxic effects on the reproductive systems of humans and other animals.
Ecological data
None
Molecular structure data
1. Molar refractive index: 50.84
2. Molar volume (cm3/mol): 154.7
3. Isotonic specific volume (90.2K ): 380.6
4. Surface tension (dyne/cm): 36.6
5. Dielectric constant (F/m): 2.71
6. Extreme Chemical rate (10-24cm3): 20.15
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 0
3. Number of hydrogen bond acceptors: 0
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 0
7. Number of heavy atoms: 12
8. Surface charge: 0
9. Complexity: 100
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
1. It is flammable, so be careful not to get close to fire sources. It is stable to heat, has similar chemical properties to benzene, and can undergo halogenation, nitration, sulfonation, hydrogenation and other reactions. For example, it reacts with bromine to form bromine derivatives. Nitrobiphenyl is produced during nitrification. Sulfonation reaction occurs with sulfuric acid in nitrobenzene to generate biphenyl-4-sulfonic acid and biphenyl-4,4'-disulfonic acid. It reacts with ozone in chloroform to form explosive tetra-ozonide. The alkylation reaction produces 4-alkylbiphenyl and 4,4'-dialkylbiphenyl. It reacts with acetyl chloride in the presence of aluminum trichloride to produce 4-acetylbiphenyl and 4,4'-diacetylbiphenyl. Use carbon disulfide as a solvent, react with oxalyl chloride and then hydrolyze to generate biphenyl-4-carboxylic acid. Chloromethylation reaction occurs with formaldehyde and hydrochloric acid in the presence of zinc chloride. 2.This product has a stimulating effect and damages the myocardium, liver and kidneys. Oral LD50 in rats: 3280mg/kg (25% olive oil solution), and in rabbits 240mg/kg . 3. Exists in flue-cured tobacco leaves, oriental tobacco leaves, and smoke.
Storage method
1. This product should be sealed and stored in a cool place.
2. This product is flammable and is in danger of burning when exposed to high temperatures, open flames, and oxidants. It should be stored in a cool, ventilated warehouse away from fire and heat sources, and stored separately from oxidants and strong acids. Pack and unload with care. Keep packaging intact.
Synthesis method
1. High-temperature coal tar contains about 3.0% biphenyl, which can be recovered from the oil wash fraction. In 1926, the American Dow Chemical Company and others began to use benzene to produce biphenyl through pyrolysis. Another source of biphenyl is a by-product of the thermal dealkylation of toluene to benzene. With the development of this process, biphenyl, a by-product of this process, has gradually become the main source of biphenyl. In the laboratory, aniline is diazotized, and the resulting diazonium salt is added to benzene. This mixture is then slowly added to the sodium hydroxide solution, and the reaction is stirred. The temperature slowly rises from below 5°C to 30-35°C. Biphenyl is generated after 8 hours of reaction.
![]()
2.Use sodium nitrite and aniline for diazotization to produce diazobenzene chloride. After neutralizing with alkali, it is condensed with benzene to obtain biphenyl, which is then distilled and refined:
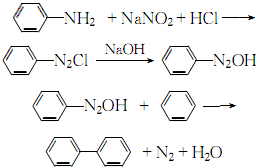
3. Tobacco: OR, 57. FC,40.
Purpose
1. Measuring the molecular weight of organic compoundsAgent, heat transfer agent, fruit antifungal agent, organic synthesis.
2. Biphenyl has high thermal stability and low vapor pressure. It has long been used as a heat transfer medium alone or mixed with diphenyl ether. Santos wax composed of biphenyl, terphenyl, etc. (containing 13% biphenyl and 61% terphenyl) can effectively absorb radiation and can be used as a heat carrier in nuclear power plants. Terphenyl ([91-94-4]) happens to be a by-product of the thermal decomposition of benzene to produce biphenyl. Depending on the reaction conditions, the ratio of biphenyl and terphenyl in the reaction product changes within a certain range. Under normal circumstances , biphenyl:terphenyl=7-8:1. Biphenyl is also used as a dyeing carrier, and its derivatives ethylbiphenyl, diethylbiphenyl, and triethylbiphenyl can be used as advanced solvents for dye solvents in pressure-sensitive copy paper. Para-phenylphenol derived from biphenyl is also mainly used to make resins. It is used as a microcapsule material for developer and coupler in the production of pressure-sensitive copy paper, and can also be used to make special coatings. Biphenyl is used as an impregnating agent for citrus wrappers and as a treatment for certain diseases of citrus plants. Chlorinated biphenyl is a plasticizer for chlorinated rubber and vinyl polymers.
3.Used in the preparation of pressure-sensitive copy paper, special coatings and other organic synthesis. Used as heat transfer agent. Chromatographic analysis reference substances and fruit fungicides.
4. A small amount is used in daily flavors and food flavors, often used as heat exchange agents and intermediates in organic synthesis.
5. This product is a better organic heat carrier and a raw material for making high-quality insulating liquids; it is used as a plasticizer and preservative, and is also used in the manufacture of dyes, engineering plastics, high-energy fuels, and anti-corrosive materials. 4-Phenylbenzophenone, the intermediate of fungal medicine, etc.
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/bis3-dimethylaminopropyl-N-CAS-33329-35-0-Tris3-dimethylaminopropylamine.pdf
extended-reading:https://www.bdmaee.net/fascat4200-catalyst-dibutyltin-diacetate-arkema-pmc/
extended-reading:https://www.newtopchem.com/archives/category/products/page/134
extended-reading:https://www.newtopchem.com/archives/39954
extended-reading:https://www.bdmaee.net/wp-content/uploads/2019/10/1-2-1.jpg
extended-reading:https://www.newtopchem.com/archives/44547
extended-reading:https://www.newtopchem.com/archives/1161
extended-reading:https://www.cyclohexylamine.net/dimethylcyclohexylamine-dmcha/
extended-reading:https://www.cyclohexylamine.net/bis2dimethylaminoethylether-22%e2%80%b2-oxybisnn-dimethylethylamine/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/37-5.jpg




















Comments