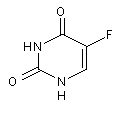
Structural formula
| Business number | 014L |
|---|---|
| Molecular formula | C4H3FN2O2 |
| Molecular weight | 130.08 |
| label |
Bifurfuridine, 5-Fluorouracil, 5-fluoro-2,4(1H,3H)-pyrimidinedione, Fluorouracil, 2,4-dihydroxy-5-fluoropyrimidine, 5-FU, Fluorouracil (USAN), 5-Fluoropyrimidine-2,4(1H,3H)-dione, 5-Fluoro-2,4-dihydroxypyrimidine, 2,4-Dihydroxy-5-fluoropyrimidine, 5-Fluor, anti-cancer raw materials, Pharmaceutical intermediates |
Numbering system
CAS number:51-21-8
MDL number:MFCD00006018
EINECS number:200-085-6
RTECS number:YR0350000
BRN number:127172
PubChem number:24278439
Physical property data
1. Properties: white crystal or powder.
2. Density (g/mL, 25/4℃): Undetermined
3. Relative vapor density (g/mL, air=1): Undetermined
4. Melting point (ºC): 282~283℃ (decomposition).
5. Boiling point (ºC, normal pressure): Undetermined
6. Boiling point (ºC, 5.2kPa): Undetermined
7. Refractive index: Undetermined
8. Flash point (ºC): Undetermined
9. Specific rotation (º): Undetermined
10. Autoignition point or ignition Combustion temperature (ºC): Undetermined
11. Vapor pressure (kPa, 25ºC): Undetermined
12. Saturated vapor pressure (kPa, 60ºC): Undetermined
13. Heat of combustion (KJ/mol): Undetermined
14. Critical temperature (ºC): Undetermined
15. Critical pressure (KPa): Undetermined
16. Logarithmic value of oil-water (octanol/water) partition coefficient: Undetermined
17. Explosion upper limit (%, V/V): Undetermined
18. Lower explosion limit (%, V/V): Undetermined
19. Solubility: Soluble in water, slightly soluble in ethanol. Almost insoluble in chloroform, soluble in dilute hydrochloric acid or sodium hydroxide solution.
Toxicological data
None
Ecological data
None
Molecular structure data
1. Molar refractive index: 25.85
2. Molar volume (cm3/mol): 84.5
3. Isotonic specific volume (90.2K): 220.4
4. Surface Tension (dyne/cm): 46.1
5. Polarizability (10-24cm3): 10.24
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 2
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 0
5. Number of tautomers: 9
6. Topological molecule polar surface area 58.2
7. Number of heavy atoms: 9
8. Surface charge: 0
9. Complexity: 199
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
None
Storage method
This product should be kept sealed and dry.
Synthesis method
1. Obtained from the condensation, cyclization and hydrolysis of ethyl fluoroacetate. 1. Condensation and cyclization. Put the sodium methoxide methanol solution into a dry stainless steel reaction pot, stir and concentrate under reduced pressure until the sodium methoxide becomes white powder, cool to 50°C, add toluene, then cool to below 10°C, add ethyl formate dropwise ester. After the addition is completed, add ethyl fluoroacetate dropwise while still keeping the temperature below 10°C. After the addition is completed, stir the reaction at about 30°C for 8 hours. Let it stand until you get a light yellow thick mixture. Add methanol and methylisourea sulfate to the condensate, stir and heat to 66-70°C for reflux reaction for 6 hours. The methanol is recovered under normal pressure until the reactant becomes a thin paste, and then evaporated under reduced pressure until it becomes viscous. Add water, heat to dissolve, and add activated carbon. Filter, acidify the filtrate with concentrated hydrochloric acid to pH 3-4, precipitate crystals, cool and filter, wash the filter cake with cold water, slurry it with boiling water and soak it, filter, wash with cold water, and dry to obtain 5-fluoro-4-hydroxy-2- Alloxypyrimidine (C5H5FN2O2). 2. Hydrolyze the above cyclization product 5-fluoro-4-hydroxy-2-methoxypyrimidine into 20% hydrochloric acid, hydrolyze at 60°C for 4 hours, and obtain 5-fluorouracil after post-treatment.
Purpose
1. Oncology drugs. It has certain curative effects on a variety of tumors such as digestive tract tumors, breast cancer, ovarian cancer, chorioepithelial cancer, cervical cancer, liver cancer, bladder cancer, skin cancer (topical application), vulvar leukoplakia (topical application), etc. Adverse reactions mainly include bone marrow transplantation and gastrointestinal reactions. Severe cases may have diarrhea and phlebitis at the local injection site, and a few may have neurological reactions such as cerebellar degeneration and ataxia. Blood pictures should be checked strictly during medication.
extended-reading:https://www.bdmaee.net/niax-a-337-delayed-tertiary-amine-catalyst-momentive/
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/67.jpg
extended-reading:https://www.morpholine.org/high-efficiency-reactive-foaming-catalyst/
extended-reading:https://www.cyclohexylamine.net/main-8/
extended-reading:https://www.bdmaee.net/fomrez-ul-24-catalyst-momentive/
extended-reading:https://www.bdmaee.net/niax-a-107-delayed-amine-catalyst-momentive/
extended-reading:https://www.bdmaee.net/sponge-hardener/
extended-reading:https://www.bdmaee.net/polyurethane-amine-catalyst-9727/
extended-reading:https://www.cyclohexylamine.net/bismuth-metal-carboxylate-catalyst-catalyst-dabco-mb20/
extended-reading:https://www.bdmaee.net/niax-dmdee-catalysts-di-morpholine-diethyl-ether-momentive/




















Comments