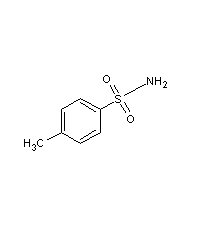
Structural formula
| Business number | 01G8 |
|---|---|
| Molecular formula | C7H9NO2S |
| Molecular weight | 171.22 |
| label |
4-Toluenesulfonamide, To sulfonamide, Toluene-4-sulfonamide, Toluenesulfonamide, p-sulfonamide toluene, 4-Toluene sulfonamide, On sulfonamide, Toluene-4-sulfonamide, Toluene sulfonamide, On ammonia toluene sulfonyl, Toughening agent, aromatic sulfur compounds |
Numbering system
CAS number:70-55-3
MDL number:MFCD00011692
EINECS number:200-741-1
RTECS number:XT5075000
BRN number:472689
PubChem number:24854164
Physical property data
1. Character: White flake or leaf-like crystal
2. Density (g/mL, 25/4℃): Uncertain
3. Relative vapor density ( g/mL, air=1): Uncertain
4. Melting point (ºC): 138.5~139
5. Boiling point (ºC, normal pressure): Uncertain
6. Boiling point (ºC, 10mmHg): 221
7. Refractive index: Uncertain
8. Flash point (ºC): 202
9. Specific rotation (º): Uncertain
10. Autoignition point or ignition temperature (ºC): Uncertain
11. Vapor pressure (kPa, 25ºC): Uncertain
12. Saturated vapor pressure (kPa, 60ºC): Uncertain
13. Heat of combustion (KJ/mol): Uncertain
14. Critical temperature (ºC): Uncertain
15. Critical pressure (KPa): Uncertain
16 . The logarithmic value of the oil-water (octanol/water) partition coefficient: Uncertain
17. Explosion upper limit (%, V/V): Uncertain
18. Explosion lower limit (% ,V/V): Uncertain
19. Solubility: Soluble in ethanol, insoluble in water (0.32 g/100 mL, 25 º C) and ether.
Toxicological data
Acute toxicity: mouse intraperitoneal LC50: 250 mg/kg; subcutaneous injection of LDLo in guinea pigs: 2 mg/kg; oral LD50 of wild birds: 75 mg/kg; mutagenicity: Salmonella microbial changes test system: 40 umol/plate;
Ecological data
None
Molecular structure data
1. Molar refractive index: 43.81
2. Molar volume (cm3/mol): 134.6
3. Isotonic specific volume (90.2K ): 351.2
4. Surface tension (dyne/cm): 46.3
5. Polarizability (10-24cm3): 17.36
Compute chemical data
1. Reference value for hydrophobic parameter calculation (XlogP): None
2. Number of hydrogen bond donors: 1
3. Number of hydrogen bond acceptors: 3
4. Number of rotatable chemical bonds: 1
5. Number of tautomers: none
6. Topological molecule polar surface area 68.5
7. Number of heavy atoms: 11
8. Surface charge: 0
9. Complexity: 209
10. Number of isotope atoms: 0
11. Determine the number of atomic stereocenters: 0
12. Uncertain number of atomic stereocenters: 0
13. Determine the number of chemical bond stereocenters: 0
14. Number of uncertain chemical bond stereocenters: 0
15. Number of covalent bond units: 1
Properties and stability
Storage method
1. Packed in an inner plastic bag, an outer woven bag or an inner plastic bag, a middle layer of kraft paper bag, and an outer woven bag. Handle it with care during storage and transportation, and protect it from moisture and heat. Store and transport according to regulations for flammable and toxic substances. 2. This product should be stored in a sealed, cool and dry place.
Synthesis method
1. Obtained from the reaction of p-toluenesulfonyl chloride and ammonia. 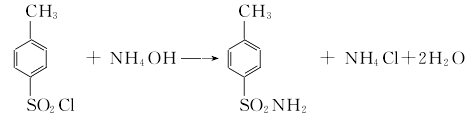 Process flow: first put an appropriate amount of ice water into the amination pot, and then mix it according to the mass Ratio (p-toluenesulfonyl chloride: pure ammonia = 1:0.2). Put the measured p-toluenesulfonyl chloride and ammonia water into the pot in sequence, start the stirrer, and use the cold liquid in the amination pot jacket to control the temperature in the pot to about 70°C. (This reaction generates a lot of heat), then lower the temperature to about 30°C and discharge the material. Put the amination into a filter bucket and filter, wash with warm water, and blot dry to obtain solid powdery crude p-toluenesulfonamide. Crude p-toluenesulfonamide contains a small amount of o-toluene sulfonamide by-product, oily colored substance. The purpose of purification and refining can be achieved by utilizing the property of para-amine to be easily soluble in sodium hydroxide solution and using activated carbon for decolorization. The material mass ratio is crude para-amine: 30% sodium hydroxide, liquid alkali: water = 100: 45: 1300; crude para-amine: activated carbon = 100: (2.5-3.5). Put the water and caustic soda into the purification pot according to the formula amount, open the jacketed steam valve and heat it to 70°C, then add the formula amount of crude para-amine, start the rear stirrer, and wait until the crude para-amine is completely dissolved. Add the formula amount of activated carbon, continue stirring for 0.5h, put the material liquid into the filter barrel, filter it with heat, wash it with hot water, and suck it dry. The filtrate is immediately poured into the refining pot, neutralized with hydrochloric acid to PH value = 2-3, and cooled to about 30-35°C. The material liquid is put into a filter barrel to filter, then washed with water until neutral, moved to a centrifuge and centrifuged to dry, that is It is a finished product containing 10% water. If a dry product is required, it is sent to an air flow dryer for drying to obtain a finished p-toluenesulfonamide product containing 1% water.
Process flow: first put an appropriate amount of ice water into the amination pot, and then mix it according to the mass Ratio (p-toluenesulfonyl chloride: pure ammonia = 1:0.2). Put the measured p-toluenesulfonyl chloride and ammonia water into the pot in sequence, start the stirrer, and use the cold liquid in the amination pot jacket to control the temperature in the pot to about 70°C. (This reaction generates a lot of heat), then lower the temperature to about 30°C and discharge the material. Put the amination into a filter bucket and filter, wash with warm water, and blot dry to obtain solid powdery crude p-toluenesulfonamide. Crude p-toluenesulfonamide contains a small amount of o-toluene sulfonamide by-product, oily colored substance. The purpose of purification and refining can be achieved by utilizing the property of para-amine to be easily soluble in sodium hydroxide solution and using activated carbon for decolorization. The material mass ratio is crude para-amine: 30% sodium hydroxide, liquid alkali: water = 100: 45: 1300; crude para-amine: activated carbon = 100: (2.5-3.5). Put the water and caustic soda into the purification pot according to the formula amount, open the jacketed steam valve and heat it to 70°C, then add the formula amount of crude para-amine, start the rear stirrer, and wait until the crude para-amine is completely dissolved. Add the formula amount of activated carbon, continue stirring for 0.5h, put the material liquid into the filter barrel, filter it with heat, wash it with hot water, and suck it dry. The filtrate is immediately poured into the refining pot, neutralized with hydrochloric acid to PH value = 2-3, and cooled to about 30-35°C. The material liquid is put into a filter barrel to filter, then washed with water until neutral, moved to a centrifuge and centrifuged to dry, that is It is a finished product containing 10% water. If a dry product is required, it is sent to an air flow dryer for drying to obtain a finished p-toluenesulfonamide product containing 1% water. 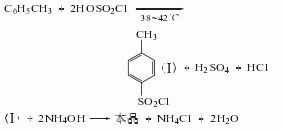 2. For p-toluenesulfonyl chloride and Pure ammonia was added to the ice water in sequence, and the mass ratio of p-toluenesulfonyl chloride and pure ammonia was controlled to be 1:0.2. The reaction was carried out under stirring, and the temperature was cooled and controlled to 70°C.
2. For p-toluenesulfonyl chloride and Pure ammonia was added to the ice water in sequence, and the mass ratio of p-toluenesulfonyl chloride and pure ammonia was controlled to be 1:0.2. The reaction was carried out under stirring, and the temperature was cooled and controlled to 70°C. 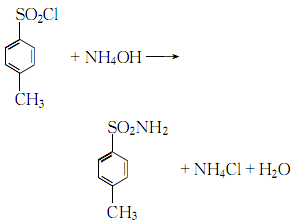 After the reaction is completed, After cooling, filtering and washing, the crude product is obtained. The crude product is subjected to alkali washing, decolorization, water washing, neutralization, filtration, water washing and drying to obtain the finished product.
After the reaction is completed, After cooling, filtering and washing, the crude product is obtained. The crude product is subjected to alkali washing, decolorization, water washing, neutralization, filtration, water washing and drying to obtain the finished product.
Purpose
1. Used in organic synthesis. p-Toluenesulfonamide is mainly used in the synthesis of chloramine-T and chloramphenicol (Tevenel). 2. Used for synthesizing fluorescent dyes, manufacturing plasticizers, synthetic resins, coatings, disinfectants and wood processing brighteners, etc. 3. Mainly used as a toughening agent for the synthesis of water-soluble melamine formaldehyde resin. 4.Used as primary brightener in bright nickel plating. Used for bright multi-layer nickel plating to make the coating bright and uniform. The general dosage is 0.2~0.3g/L.
extended-reading:https://www.newtopchem.com/archives/538
extended-reading:https://www.bdmaee.net/wp-content/uploads/2022/08/-25-S-Lupragen-N202-TEDA-L25B.pdf
extended-reading:https://www.newtopchem.com/archives/44038
extended-reading:https://www.bdmaee.net/nt-cat-t45-catalyst-cas121-143-5-newtopchem/
extended-reading:https://www.newtopchem.com/archives/42992
extended-reading:https://www.newtopchem.com/archives/44937
extended-reading:https://www.newtopchem.com/archives/category/products/page/92
extended-reading:https://www.morpholine.org/category/morpholine/page/9/
extended-reading:https://www.newtopchem.com/archives/968
extended-reading:https://www.newtopchem.com/archives/1037




















Comments